Ca2+/calmodulin-dependent protein kinase II-dependent remodeling of Ca2+ current in pressure overload heart failure
- PMID: 18622016
- PMCID: PMC2533065
- DOI: 10.1074/jbc.M803043200
Ca2+/calmodulin-dependent protein kinase II-dependent remodeling of Ca2+ current in pressure overload heart failure
Abstract
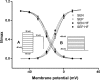
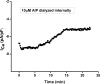
Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) activity is increased in heart failure (HF), a syndrome characterized by markedly increased risk of arrhythmia. Activation of CaMKII increases peak L-type Ca(2+) current (I(Ca)) and slows I(Ca) inactivation. Whether these events are linked mechanistically is unknown. I(Ca) was recorded in acutely dissociated subepicardial and subendocardial murine left ventricular (LV) myocytes using the whole cell patch clamp method. Pressure overload heart failure was induced by surgical constriction of the thoracic aorta. I(Ca) density was significantly larger in subepicardial myocytes than in subendocardial/myocytes. Similar patterns were observed in the cell surface expression of alpha1c, the channel pore-forming subunit. In failing LV, I(Ca) density was increased proportionately in both cell types, and the time course of I(Ca) inactivation was slowed. This typical pattern of changes suggested a role of CaMKII. Consistent with this, measurements of CaMKII activity revealed a 2-3-fold increase (p < 0.05) in failing LV. To test for a causal link, we measured frequency-dependent I(Ca) facilitation. In HF myocytes, this CaMKII-dependent process could not be induced, suggesting already maximal activation. Internal application of active CaMKII in failing myocytes did not elicit changes in I(Ca). Finally, CaMKII inhibition by internal diffusion of a specific peptide inhibitor reduced I(Ca) density and inactivation time course to similar levels in control and HF myocytes. I(Ca) density manifests a significant transmural gradient, and this gradient is preserved in heart failure. Activation of CaMKII, a known pro-arrhythmic molecule, is a major contributor to I(Ca) remodeling in load-induced heart failure.
Figures








References
-
- Janse, M. J. (2004) Cardiovasc. Res. 61 208-217 - PubMed
-
- Akar, F. G., and Tomaselli, G. F. (2005) Ann. Med. 37 44-54 - PubMed
-
- Heineke, J., and Molkentin, J. D. (2006) Nat. Rev. Mol. Cell. Biol. 7 589-600 - PubMed
-
- Hill, J. A., and Olson, E. N. (2008) New Engl. J. Med. 358 1370-1380 - PubMed
-
- Maier, L. S., Bers, D. M., and Brown, J. H. (2007) Cardiovasc. Res. 73 629-630 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

