Phosphorylation of endothelial nitric-oxide synthase regulates superoxide generation from the enzyme
- PMID: 18622039
- PMCID: PMC2556006
- DOI: 10.1074/jbc.M802269200
Phosphorylation of endothelial nitric-oxide synthase regulates superoxide generation from the enzyme
Abstract
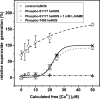
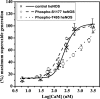
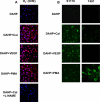
In the vasculature, nitric oxide (NO) is generated by endothelial NO synthase (eNOS) in a calcium/calmodulin-dependent reaction. With oxidative stress, the critical cofactor BH(4) is depleted, and NADPH oxidation is uncoupled from NO generation, leading to production of (O(2)*). Although phosphorylation of eNOS regulates in vivo NO generation, the effects of phosphorylation on eNOS coupling and O(2)* generation are unknown. Therefore, we phosphorylated recombinant BH(4)-free eNOS in vitro using native kinases and determined O(2)* generation using EPR spin trapping. Phosphorylation of Ser-1177 by Akt led to an increase (>50%) in maximal O(2)* generation from eNOS. Moreover, Ser-1177 phosphorylation greatly altered the Ca(2+) sensitivity of eNOS, such that O(2)* generation became largely Ca(2+)-independent. In contrast, phosphorylation of eNOS at Thr-495 by protein kinase Calpha (PKCalpha) had no effect on maximum activity or calcium sensitivity but decreased calmodulin binding and increased association with caveolin. In endothelial cells, eNOS-dependent O(2)* generation was stimulated by vascular endothelial growth factor that induced phosphorylation of Ser-1177. With PKC activation that led to phosphorylation of Thr-495, no inhibition of O(2)* generation occurred. As such, phosphorylation of eNOS at Ser-1177 is pivotal in the direct regulation of O(2)* and NO generation, altering both the Ca(2+) sensitivity of the enzyme and rate of product formation, whereas phosphorylation of Thr-495 indirectly affects this process through regulation of the calmodulin and caveolin interaction. Thus, Akt-mediated phosphorylation modulates eNOS uncoupling and greatly increases O(2)* generation from the enzyme at low Ca(2+) concentrations, and PKCalpha-mediated phosphorylation alters the sensitivity of the enzyme to other negative regulatory signals.
Figures




Similar articles
-
Suppression of eNOS-derived superoxide by caveolin-1: a biopterin-dependent mechanism.Am J Physiol Heart Circ Physiol. 2011 Sep;301(3):H903-11. doi: 10.1152/ajpheart.00936.2010. Epub 2011 Jul 1. Am J Physiol Heart Circ Physiol. 2011. PMID: 21724868 Free PMC article.
-
Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity.Circ Res. 2001 Jun 8;88(11):E68-75. doi: 10.1161/hh1101.092677. Circ Res. 2001. PMID: 11397791
-
Tannin 1-alpha-O-galloylpunicalagin induces the calcium-dependent activation of endothelial nitric-oxide synthase via the phosphatidylinositol 3-kinase/Akt pathway in endothelial cells.Mol Nutr Food Res. 2008 Oct;52(10):1162-71. doi: 10.1002/mnfr.200700335. Mol Nutr Food Res. 2008. PMID: 18435486
-
Regulation of endothelial nitric oxide synthase activity by protein-protein interaction.Curr Pharm Des. 2014;20(22):3514-20. doi: 10.2174/13816128113196660752. Curr Pharm Des. 2014. PMID: 24180383 Free PMC article. Review.
-
Molecular mechanisms underlying the activation of eNOS.Pflugers Arch. 2010 May;459(6):793-806. doi: 10.1007/s00424-009-0767-7. Epub 2009 Dec 13. Pflugers Arch. 2010. PMID: 20012875 Review.
Cited by
-
Genetic Deletion of ACE2 Induces Vascular Dysfunction in C57BL/6 Mice: Role of Nitric Oxide Imbalance and Oxidative Stress.PLoS One. 2016 Apr 12;11(4):e0150255. doi: 10.1371/journal.pone.0150255. eCollection 2016. PLoS One. 2016. PMID: 27070147 Free PMC article.
-
LncRNA HOXA11-AS promotes vascular endothelial cell injury in atherosclerosis by regulating the miR-515-5p/ROCK1 axis.ESC Heart Fail. 2022 Aug;9(4):2259-2271. doi: 10.1002/ehf2.13815. Epub 2022 May 16. ESC Heart Fail. 2022. PMID: 35578440 Free PMC article.
-
Nitric oxide, oxidative stress, and p66Shc interplay in diabetic endothelial dysfunction.Biomed Res Int. 2014;2014:193095. doi: 10.1155/2014/193095. Epub 2014 Mar 5. Biomed Res Int. 2014. PMID: 24734227 Free PMC article. Review.
-
Hypoxia and reoxygenation induce endothelial nitric oxide synthase uncoupling in endothelial cells through tetrahydrobiopterin depletion and S-glutathionylation.Biochemistry. 2014 Jun 10;53(22):3679-88. doi: 10.1021/bi500076r. Epub 2014 May 29. Biochemistry. 2014. PMID: 24758136 Free PMC article.
-
Posttranscriptional and transcriptional regulation of endothelial nitric-oxide synthase during hypoxia: the role of microRNAs.Cell Mol Biol Lett. 2016 Sep 6;21:16. doi: 10.1186/s11658-016-0017-x. eCollection 2016. Cell Mol Biol Lett. 2016. PMID: 28536619 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

