The use of exenatide in islet transplant recipients with chronic allograft dysfunction: safety, efficacy, and metabolic effects
- PMID: 18622276
- PMCID: PMC2772201
- DOI: 10.1097/TP.0b013e31817c4ab3
The use of exenatide in islet transplant recipients with chronic allograft dysfunction: safety, efficacy, and metabolic effects
Abstract
Background: A current limitation of islet transplantation is reduced long-term graft function. The glucagon-like peptide-1 receptor agonist, exenatide (Byetta, Amylin Pharmaceuticals, CA) has properties that could improve existing islet function, prevent further loss of islet mass and possibly even stimulate islet regeneration.
Methods: This prospective study evaluated the safety, efficacy, and metabolic effects of exenatide in subjects with type 1 diabetes mellitus and islet allograft dysfunction requiring exogenous insulin.
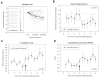
Results: Sixteen subjects commenced exenatide, 12 continue (follow-up 214+/-57 days; range 108-287), four (25%) discontinued medication because of side effects. At 6 months, exogenous insulin was significantly reduced with stable glycemic control (0.15+/-0.02 vs. 0.11+/-0.025 U/kg per day; P<0.0001); three subjects discontinued insulin from 4, 5, and 9 U/day, respectively, two sustained insulin independence with A1c reduction below graft dysfunction criteria. Postprandial capillary blood glucose was significantly decreased (129.4+/-3.8 vs. 118.7+/-4.6 mg/dL; P<0.001), C-peptide and C-peptide-to-glucose ratio increased significantly by 5th and 6th months of treatment (ratio, 1.09+/-0.15 vs. 1.52+/-0.18; P<0.05). Weight loss more than 3 kg occurred in 8 of 12 (67%) subjects. Stimulation testing demonstrated improved glucose disposal and C-peptide secretion (glucose area under the curve 52,332+/-3,219 vs. 42,072+/-1,965; P=0.002 mg x min x dL, mixed meal stimulation index 0.50+/-0.06 vs. 0.66+/-0.09; P=0.03 pmol x mL), with marked suppression of glucagon secretion and progressive increase in amylin secretion. Side effects were more frequent and severe compared with published reports in type 2 diabetes, tolerated doses were lower.
Conclusions: Exenatide was tolerated in this patient population after appropriate dose titration and there appeared to be gradual but sustained positive effects on glycemic control and islet graft function.
Conflict of interest statement
No author conflict of interest.
Figures


Comment in
-
Exenatide use in islet transplantation: words of caution.Transplantation. 2009 Jan 15;87(1):153; author reply 154. doi: 10.1097/TP.0b013e318191eb48. Transplantation. 2009. PMID: 19136906 No abstract available.
References
-
- Ricordi C. Islet transplantation: a brave new world. Diabetes. 2003;52(7):1595–603. - PubMed
-
- Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–8. - PubMed
-
- Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–30. - PubMed
-
- Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54(7):2060–9. - PubMed
-
- Froud T, Ricordi C, Baidal DA, et al. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am J Transplant. 2005;5(8):2037–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK025802/DK/NIDDK NIH HHS/United States
- 1R01 DK25802-21/DK/NIDDK NIH HHS/United States
- M01 RR016587/RR/NCRR NIH HHS/United States
- U01 DK070460/DK/NIDDK NIH HHS/United States
- 1R01 D59993-04/PHS HHS/United States
- R01 DK055347/DK/NIDDK NIH HHS/United States
- R01 DK056953/DK/NIDDK NIH HHS/United States
- U42 RR016603/RR/NCRR NIH HHS/United States
- 5 R01 DK056953/DK/NIDDK NIH HHS/United States
- 5 R01 DK55347/DK/NIDDK NIH HHS/United States
- M01RR16587/RR/NCRR NIH HHS/United States
- M01 RR005280/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

