Neurons born in the adult dentate gyrus form functional synapses with target cells
- PMID: 18622400
- PMCID: PMC2572641
- DOI: 10.1038/nn.2156
Neurons born in the adult dentate gyrus form functional synapses with target cells
Abstract
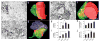
Adult neurogenesis occurs in the hippocampus and the olfactory bulb of the mammalian CNS. Recent studies have demonstrated that newborn granule cells of the adult hippocampus are postsynaptic targets of excitatory and inhibitory neurons, but evidence of synapse formation by the axons of these cells is still lacking. By combining retroviral expression of green fluorescent protein in adult-born neurons of the mouse dentate gyrus with immuno-electron microscopy, we found output synapses that were formed by labeled terminals on appropriate target cells in the CA3 area and the hilus. Furthermore, retroviral expression of channelrhodopsin-2 allowed us to light-stimulate newborn granule cells and identify postsynaptic target neurons by whole-cell recordings in acute slices. Our structural and functional evidence indicates that axons of adult-born granule cells establish synapses with hilar interneurons, mossy cells and CA3 pyramidal cells and release glutamate as their main neurotransmitter.
Figures




References
-
- Schinder AF, Gage FH. A hypothesis about the role of adult neurogenesis in hippocampal function. Physiology (Bethesda) 2004;19:253–261. - PubMed
-
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. - PubMed
-
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. - PubMed
-
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. - PubMed
-
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

