Cell type-specific regulation of DARPP-32 phosphorylation by psychostimulant and antipsychotic drugs
- PMID: 18622401
- PMCID: PMC2737705
- DOI: 10.1038/nn.2153
Cell type-specific regulation of DARPP-32 phosphorylation by psychostimulant and antipsychotic drugs
Abstract
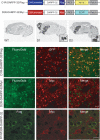
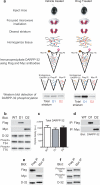
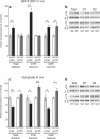
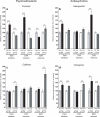
DARPP-32 is a dual-function protein kinase/phosphatase inhibitor that is involved in striatal signaling. The phosphorylation of DARPP-32 at threonine 34 is essential for mediating the effects of both psychostimulant and antipsychotic drugs; however, these drugs are known to have opposing behavioral and clinical effects. We hypothesized that these drugs exert differential effects on striatonigral and striatopallidal neurons, which comprise distinct output pathways of the basal ganglia. To directly test this idea, we developed bacterial artificial chromosome transgenic mice that allowed the analysis of DARPP-32 phosphorylation selectively in striatonigral and striatopallidal neurons. Using this new methodology, we found that cocaine, a psychostimulant, and haloperidol, a sedation-producing antipsychotic, exert differential effects on DARPP-32 phosphorylation in the two neuronal populations that can explain their opposing behavioral effects. Furthermore, we found that a variety of drugs that target the striatum have cell type-specific effects that previous methods were not able to discern.
Figures

References
-
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. - PubMed
-
- Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. - PubMed
-
- Bibb JA, et al. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402:669–671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

