Discovery of sarcosine dimethylglycine methyltransferase from Galdieria sulphuraria
- PMID: 18623062
- PMCID: PMC2742680
- DOI: 10.1002/prot.22147
Discovery of sarcosine dimethylglycine methyltransferase from Galdieria sulphuraria
Abstract
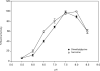
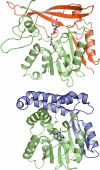
An enzyme with sarcosine dimethylglycine methyltransferase (SDMT) activity has been identified in the thermophilic eukaryote, Galdieria sulphuraria. The crystal structure of the enzyme, solved to a resolution of 1.95 A, revealed a fold highly similar to that of mycolic acid synthases. The kcat and apparent K(M) values were 64.3 min(-1) and 2.0 mM for sarcosine and 85.6 min(-1) and 2.8 mM for dimethylglycine, respectively. Apparent K(M) values of S-adenosylmethionine were 144 and 150 microM for sarcosine and dimethylglycine, respectively, and the enzyme melting temperature was 61.1 degrees C. Modeling of cofactor binding in the active site based on the structure of methoxy mycolic acid synthase 2 revealed a number of conserved interactions within the active site.
Copyright 2008 Wiley-Liss, Inc.
Figures






Similar articles
-
Characterization of glycine sarcosine N-methyltransferase and sarcosine dimethylglycine N-methyltransferase.Appl Environ Microbiol. 2001 May;67(5):2044-50. doi: 10.1128/AEM.67.5.2044-2050.2001. Appl Environ Microbiol. 2001. PMID: 11319079 Free PMC article.
-
Characterization of osmolyte betaine synthesizing sarcosine dimethylglycine N-methyltransferase from Methanohalophilus portucalensis.Arch Microbiol. 2009 Oct;191(10):735-43. doi: 10.1007/s00203-009-0501-z. Epub 2009 Aug 20. Arch Microbiol. 2009. PMID: 19693490
-
Effects of substrate and potassium on the betaine-synthesizing enzyme glycine sarcosine dimethylglycine N-methyltransferase from a halophilic methanoarchaeon Methanohalophilus portucalensis.Res Microbiol. 2006 Dec;157(10):948-55. doi: 10.1016/j.resmic.2006.08.007. Epub 2006 Oct 26. Res Microbiol. 2006. PMID: 17098399
-
SAM (dependent) I AM: the S-adenosylmethionine-dependent methyltransferase fold.Curr Opin Struct Biol. 2002 Dec;12(6):783-93. doi: 10.1016/s0959-440x(02)00391-3. Curr Opin Struct Biol. 2002. PMID: 12504684 Review.
-
Structure, function and physiological role of glycine N-methyltransferase.Int J Biochem Cell Biol. 1998 Jan;30(1):13-26. doi: 10.1016/s1357-2725(97)00105-2. Int J Biochem Cell Biol. 1998. PMID: 9597750 Review.
Cited by
-
The genomes of polyextremophilic cyanidiales contain 1% horizontally transferred genes with diverse adaptive functions.Elife. 2019 May 31;8:e45017. doi: 10.7554/eLife.45017. Elife. 2019. PMID: 31149898 Free PMC article.
-
In Vitro Protein Stability of Two Naturally Occurring Thiopurine S-Methyltransferase Variants: Biophysical Characterization of TPMT*6 and TPMT*8.ACS Omega. 2017 Aug 31;2(8):4991-4999. doi: 10.1021/acsomega.7b00801. Epub 2017 Aug 28. ACS Omega. 2017. PMID: 30023734 Free PMC article.
-
A simplified characterization of S-adenosyl-l-methionine-consuming enzymes with 1-Step EZ-MTase: a universal and straightforward coupled-assay for in vitro and in vivo setting.Chem Sci. 2017 Sep 1;8(9):6601-6612. doi: 10.1039/c7sc02830j. Epub 2017 Jul 27. Chem Sci. 2017. PMID: 29449933 Free PMC article.
-
Insights into the regulation of DMSP synthesis in the diatom Thalassiosira pseudonana through APR activity, proteomics and gene expression analyses on cells acclimating to changes in salinity, light and nitrogen.PLoS One. 2014 Apr 14;9(4):e94795. doi: 10.1371/journal.pone.0094795. eCollection 2014. PLoS One. 2014. PMID: 24733415 Free PMC article.
-
Characterization and regulation of the osmolyte betaine synthesizing enzymes GSMT and SDMT from halophilic methanogen Methanohalophilus portucalensis.PLoS One. 2011;6(9):e25090. doi: 10.1371/journal.pone.0025090. Epub 2011 Sep 20. PLoS One. 2011. PMID: 21949863 Free PMC article.
References
-
- Schliess F, Haussinger D. The cellular hydration state: a critical determinant for cell death and survival. Biol Chem. 2002;383(3-4):577–583. - PubMed
-
- Finkelstein JD, Martin JJ, Harris BJ, Kyle WE. Regulation of hepatic betaine-homocysteine methyltransferase by dietary betaine. J Nutr. 1983;113(3):519–521. - PubMed
-
- Santoro MM, Liu Y, Khan SM, Hou LX, Bolen DW. Increased thermal stability of proteins in the presence of naturally occurring osmolytes. Biochemistry. 1992;31(23):5278–5283. - PubMed
-
- Ladas NP, Papageorgiou GC. Cell turgor: A critical factor for the proliferation of cyanobacteria at unfavorable salinity. Photosynth Res. 2000;65(2):155–164. - PubMed
-
- Sakamoto A, Murata N. The role of glycine betaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ. 2002;25(2):163–171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

