Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis
- PMID: 18623174
- PMCID: PMC2597552
- DOI: 10.1002/ibd.20520
Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis
Abstract
Background: The Mayo score and a noninvasive 9-point partial Mayo score are used as outcome measures for clinical trials assessing therapy for ulcerative colitis (UC). There are limited data assessing what defines a clinically relevant change in these indices. We sought to assess what constitutes a clinically meaningful change in these indices using data from a recently completed placebo-controlled clinical trial.
Methods: In all, 105 patients were enrolled in a 12-week randomized, placebo-controlled trial assessing rosiglitazone for treatment of mild to moderate UC. We compared the change in the Mayo score, the partial Mayo score, and a 6-point score composed just of the stool frequency and bleeding components of the Mayo score to the patient's perception of disease activity at week 0 and week 12. Optimal cutpoints were calculated as the maximal product of sensitivity and specificity.
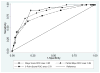
Results: Each index was strongly correlated with the patient's rating of disease activity at week 12 (Spearman correlations 0.61-0.71, P < 0.0001 for all correlations). The maximal product of sensitivity and specificity to identify patient reported improvement of disease activity was achieved using cutpoints for change of 2.5 for the Mayo score (sensitivity 88%, specificity 80%), 2.5 for the partial Mayo score (sensitivity 88%, specificity 87%), and 1.5 for the 6-point score (sensitivity 88%, specificity 80%).
Conclusions: The partial Mayo score and the 6-point score composed solely of the stool frequency and bleeding components performed as well as the full Mayo score to identify patient perceived clinical response.
Trial registration: ClinicalTrials.gov NCT00065065.
Conflict of interest statement
The authors report no potential conflicts of interest related to this study.
Figures

References
-
- D’Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763–86. - PubMed
-
- Higgins PD, Schwartz M, Mapili J, Zimmermann EM. Is endoscopy necessary for the measurement of disease activity in ulcerative colitis? American Journal of Gastroenterology. 2005;100:355–61. - PubMed
-
- Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine. 2005;353:2462–76. - PubMed
-
- Lewis JD, Lichtenstein GR, Stein RB, et al. An open-label trial of the PPAR-gamma ligand rosiglitazone for active ulcerative colitis. American Journal of Gastroenterology. 2001;96:3323–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
