Heterogeneity of kinase inhibitor resistance mechanisms in GIST
- PMID: 18623623
- PMCID: PMC2693040
- DOI: 10.1002/path.2382
Heterogeneity of kinase inhibitor resistance mechanisms in GIST
Abstract
Most GIST patients develop clinical resistance to KIT/PDGFRA tyrosine kinase inhibitors (TKI). However, it is unclear whether clinical resistance results from single or multiple molecular mechanisms in each patient. KIT and PDGFRA mutations were evaluated in 53 GIST metastases obtained from 14 patients who underwent surgical debulking after progression on imatinib or sunitinib. To interrogate possible resistance mechanisms across a broad biological spectrum of GISTs, inter- and intra-lesional heterogeneity of molecular drug-resistance mechanisms were evaluated in the following: conventional KIT (CD117)-positive GISTs with KIT mutations in exon 9, 11 or 13; KIT-negative GISTs; GISTs with unusual morphology; and KIT/PDGFRA wild-type GISTs. Genomic KIT and PDGFRA mutations were characterized systematically, using complementary techniques including D-HPLC for KIT exons 9, 11-18 and PDGFRA exons 12, 14, 18, and mutation-specific PCR (V654A, D820G, N822K, Y823D). Primary KIT oncogenic mutations were found in 11/14 patients (79%). Of these, 9/11 (83%), had secondary drug-resistant KIT mutations, including six (67%) with two to five different secondary mutations in separate metastases, and three (34%) with two secondary KIT mutations in the same metastasis. The secondary mutations clustered in the KIT ATP binding pocket and kinase catalytic regions. FISH analyses revealed KIT amplicons in 2/10 metastases lacking secondary KIT mutations. This study demonstrates extensive intra- and inter-lesional heterogeneity of resistance mutations and gene amplification in patients with clinically progressing GIST. KIT kinase resistance mutations were not found in KIT/PDGFRA wild-type GISTs or in KIT-mutant GISTs showing unusual morphology and/or loss of KIT expression by IHC, indicating that resistance mechanisms are fundamentally different in these tumours. Our observations underscore the heterogeneity of clinical TKI resistance, and highlight the therapeutic challenges involved in salvaging patients after clinical progression on TKI monotherapies.
Conflict of interest statement
No conflicts of interest were declared.
Figures

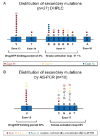
 ) or detected by both AS–PCR and D-HPLC (●) in 18 tumour samples. Associated primary KIT mutations are indicated in red (exon 11 mutations), yellow (exon 9 mutations) and green (exon 13 mutations)
) or detected by both AS–PCR and D-HPLC (●) in 18 tumour samples. Associated primary KIT mutations are indicated in red (exon 11 mutations), yellow (exon 9 mutations) and green (exon 13 mutations)
References
-
- Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21(23):4342–4349. - PubMed
-
- Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299(5607):708–710. - PubMed
-
- Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–580. - PubMed
-
- Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472–480. - PubMed
-
- Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364(9440):1127–1134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

