Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity
- PMID: 18624726
- PMCID: PMC2632685
- DOI: 10.1111/j.1365-2567.2008.02892.x
Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity
Abstract
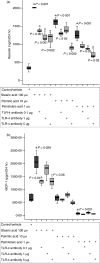
To study the effects of fatty acids and the involvement of the Toll-like receptor-4/nuclear factor-kappaB (TLR-4/NF-kappaB) pathway with respect to the secretion of adipokines from adipocytes 3T3-L1 adipocytes were stimulated with increasing doses of fatty acids. The secretion of adiponectin, resistin and monocyte chemoattractant protein-1 (MCP-1) was measured by enzyme-linked immunosorbent assay. The NF-kappaB p65 nuclear translocation and TLR-4 expression were investigated by Western blot. The effects mediated by NF-kappaB were tested using a specific NF-kappaB-inhibitor and TLR-4-induced effects were analysed with a neutralizing TLR-4 antibody. Binding of (14)C-labelled fatty acids to TLR-4/MD-2 was investigated using a FLAG-tagged extracellular part of TLR-4 fused to full-length MD-2 via a linker (lipopolysaccharide-Trap). The messenger RNA (mRNA) expression of adipokines in abdominal adipose tissue of rats fed a standard chow or a high-fat diet was investigated by reverse transcription-polymerase chain reaction. The TLR-4 is induced during adipocyte differentiation and its expression is enhanced following fatty acid stimulation. The stimulatory effects of stearic and palmitic acids on MCP-1 secretion and of palmitoleic acid on resistin secretion are mediated via NF-kappaB. The stimulatory effects of stearic, palmitic and palmitoleic acids on resistin secretion and the stimulatory effect of stearic acid on MCP-1 secretion are mediated via TLR-4. Fatty acid-mediated effects are caused by an endogenous ligand because fatty acids were shown not to bind directly to TLR-4/MD-2. Adipose tissue mRNA expression and serum levels of adipokines did not differ in rats fed a high-fat diet. These data provide a new molecular mechanism by which fatty acids can link nutrition with innate immunity.
Figures


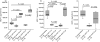



References
-
- Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB. Adiponectin – a key adipokine in the metabolic syndrome. Diabetes Obes Metab. 2006;8:264–80. - PubMed
-
- Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci (Lond) 2006;110:267–78. - PubMed
-
- Vasseur F. Adiponectin and its receptors: partners contributing to the “vicious circle” leading to the metabolic syndrome? Pharmacol Res. 2006;53:478–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

