Neuronal cell death in neurodegenerative diseases: recurring themes around protein handling
- PMID: 18624755
- PMCID: PMC4514105
- DOI: 10.1111/j.1582-4934.2008.00402.x
Neuronal cell death in neurodegenerative diseases: recurring themes around protein handling
Abstract
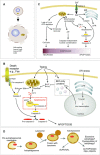
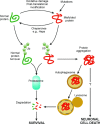
Neuronal cell death plays a role in many chronic neurodegenerative diseases with the loss of particular subsets of neurons. The loss of the neurons occurs during a period of many years, which can make the mode(s) of cell death and the initiating factors difficult to determine. In vitro and in vivo models have proved invaluable in this regard, yielding insight into cell death pathways. This review describes the main mechanisms of neuronal cell death, particularly apoptosis, necrosis, excitotoxicity and autophagic cell death, and their role in neurodegenerative diseases such as ischaemia, Alzheimer's, Parkinson's and Huntington's diseases. Crosstalk between these death mechanisms is also discussed. The link between cell death and protein mishandling, including misfolded proteins, impairment of protein degradation, protein aggregation is described and finally, some pro-survival strategies are discussed.
Figures

References
-
- Yankner BA, Lu T, Loerch P. The aging brain. Annu Rev Pathol. 2008;3:41–66. - PubMed
-
- Stroh C, Schulze-Osthoff K. Death by a thousand cuts: an ever increasing list of caspase substrates. Cell Death Differ. 1998;5:997–1000. - PubMed
-
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-depend-ent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–89. - PubMed
-
- Zou H, Li Y, Liu X, Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates pro-caspase-9. J Biol Chem. 1999;274:11549–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

