Parenchymal and vascular Abeta-deposition and its effects on the degeneration of neurons and cognition in Alzheimer's disease
- PMID: 18624777
- PMCID: PMC4506155
- DOI: 10.1111/j.1582-4934.2008.00411.x
Parenchymal and vascular Abeta-deposition and its effects on the degeneration of neurons and cognition in Alzheimer's disease
Abstract
The deposition of the amyloid beta-protein (Abeta) is one of the pathological hallmarks of Alzheimer's disease (AD). Abeta-deposits show the morphology of senile plaques and cerebral amyloid angiopathy (CAA). Senile plaques and vascular Abeta-deposits occur first in neocorti-cal areas. Then, they expand hierarchically into further brain regions. The distribution of Abeta plaques throughout the entire brain, thereby correlates with the clinical status of the patients. Imaging techniques for Abeta make use of the hierarchical distribution of Abeta to distinguish AD patients from non-AD patients. However, pathology seen in AD patients represents a late stage of a pathological process starting 10-30 years earlier in cognitively normal individuals. In addition to the fibrillar amyloid of senile plaques, oligomeric and monomeric Abeta is found in the brain. Recent studies revealed that oligomeric Abeta is presumably the most toxic Abeta-aggregate, which interacts with glutamatergic synapses. In doing so, dendrites are presumed to be the primary target for Abeta-toxicity. In addition, vascular Abeta-deposits can lead to capillary occlusion and blood flow disturbances presumably contributing to the alteration of neurons in addition to the direct neurotoxic effects of Abeta. All these findings point to an important role of Abeta and its aggregates in the neurodegenerative process of AD. Since there is already significant neuron loss in AD patients, treatment strategies aimed at reducing the amyloid load will presumably not cure the symptoms of dementia but they may stop disease progression. Therefore, it seems to be necessary to protect the brain from Abeta-toxicity already in stages of the disease with minor neuron loss before the onset of cognitive symptoms.
Figures


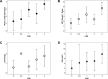
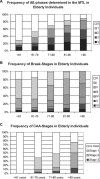
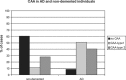
References
-
- Alzheimer A. Ueber eine eigenartige Erkrankung der Hirnrinde. Allg Zschr Psych. 1907;64:146–8.
-
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–64. - PubMed
-
- Masliah E, Mallory M, Hansen L, DeTeresa R, Alford M, Terry R. Synaptic and neuritic alterations during the progression of Alzheimer's disease. Neurosci Lett. 1994;174:67–72. - PubMed
-
- Terry RD, Peck A, DeTeresa R, Schechter R, Horoupian DS. Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann Neurol. 1981;10:184–92. - PubMed
-
- Duyckaerts C, Dickson DW. Neuro -pathology of Alzheimer's disease. In: Dickson D, editor. Neurodegeneration: the molecular pathology of dementia and movement disorders. ISN Neuropath Press; 2003. pp. 47–65. In:, editor.. Basel:.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

