Evaluation of immunoglobulin purification methods and their impact on quality and yield of antigen-specific antibodies
- PMID: 18625058
- PMCID: PMC2490700
- DOI: 10.1186/1475-2875-7-129
Evaluation of immunoglobulin purification methods and their impact on quality and yield of antigen-specific antibodies
Abstract
Background: Antibodies are the main effectors against malaria blood-stage parasites. Evaluation of functional activities in immune sera from Phase 2a/b vaccine trials may provide invaluable information in the search for immune correlates of protection. However, the presence of anti-malarial-drugs, improper collection/storage conditions or concomitant immune responses against other pathogens can contribute to non-specific anti-parasite activities when the sera/plasma are tested in vitro. Purification of immunoglobulin is a standard approach for reducing such non-specific background activities, but the purification method itself can alter the quality and yield of recovered Ag-specific antibodies.
Methods: To address this concern, various immunoglobulin (Ig) purification methods (protein G Sepharose, protein A/G Sepharose, polyethylene glycol and caprylic acid-ammonium sulphate precipitation) were evaluated for their impact on the quality, quantity and functional activity of purified rabbit and human Igs. The recovered Igs were analysed for yield and purity by SDS-PAGE, for quality by Ag-specific ELISAs (determining changes in titer, avidity and isotype distribution) and for functional activity by in vitro parasite growth inhibition assay (GIA).
Results: This comparison demonstrated that overall polyethylene glycol purification of human serum/plasma samples and protein G Sepharose purification of rabbit sera are optimal for recovering functional Ag-specific antibodies.
Conclusion: Consequently, critical consideration of the purification method is required to avoid selecting non-representative populations of recovered Ig, which could influence interpretations of vaccine efficacy, or affect the search for immune correlates of protection.
Figures


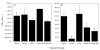


Similar articles
-
Anti-immunoglobulin antisera used in an ELISA to detect antibodies in barramundi Lates calcarifer to Cryptocaryon irritans.Dis Aquat Organ. 1999 Apr 15;36(1):21-8. doi: 10.3354/dao036021. Dis Aquat Organ. 1999. PMID: 10349549
-
Comparative studies on the purity and specificity of yolk immunoglobulin Y isolated from eggs laid by hens immunized with Toxoplasma gondii antigen.Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Dec;267(2):247-53. doi: 10.1016/s0176-6724(87)80009-3. Zentralbl Bakteriol Mikrobiol Hyg A. 1987. PMID: 3128923
-
Plasmodium yoelii blood-stage antigens newly identified by immunoaffinity using purified IgG antibodies from malaria-resistant mice.Immunobiology. 2012 Aug;217(8):823-30. doi: 10.1016/j.imbio.2012.05.002. Epub 2012 May 11. Immunobiology. 2012. PMID: 22658767
-
A comparison between different chemical fractionation methods for immunoglobulin preparation.J Immunoassay Immunochem. 2025 Mar 4;46(2):169-185. doi: 10.1080/15321819.2025.2450664. Epub 2025 Jan 11. J Immunoassay Immunochem. 2025. PMID: 39799401
-
Immunity to recombinant plasmodium falciparum merozoite surface protein 1 (MSP1): protection in Aotus nancymai monkeys strongly correlates with anti-MSP1 antibody titer and in vitro parasite-inhibitory activity.Infect Immun. 2006 Aug;74(8):4573-80. doi: 10.1128/IAI.01679-05. Infect Immun. 2006. PMID: 16861644 Free PMC article.
Cited by
-
Sensitivity of lateral flow technique for diagnosis of canine parvovirus.Sci Rep. 2024 Mar 1;14(1):5060. doi: 10.1038/s41598-024-55548-x. Sci Rep. 2024. PMID: 38424259 Free PMC article.
-
Novel Antibody-Peptide Binding Assay Indicates Presence of Immunoglobulins against EGFR Phospho-Site S1166 in High-Grade Glioma.Int J Mol Sci. 2022 May 2;23(9):5061. doi: 10.3390/ijms23095061. Int J Mol Sci. 2022. PMID: 35563452 Free PMC article.
-
Strain-specific Plasmodium falciparum growth inhibition among Malian children immunized with a blood-stage malaria vaccine.PLoS One. 2017 Mar 10;12(3):e0173294. doi: 10.1371/journal.pone.0173294. eCollection 2017. PLoS One. 2017. PMID: 28282396 Free PMC article. Clinical Trial.
-
Allergen-specific IgG antibodies purified from mite-allergic patients sera block the IgE recognition of Dermatophagoides pteronyssinus antigens: an in vitro study.Clin Dev Immunol. 2013;2013:657424. doi: 10.1155/2013/657424. Epub 2013 Aug 28. Clin Dev Immunol. 2013. PMID: 24069042 Free PMC article.
-
Development of a New Indirect ELISA Test for the Detection of Anti-Feline Coronavirus Antibodies in Cats.Vet Sci. 2025 Mar 4;12(3):245. doi: 10.3390/vetsci12030245. Vet Sci. 2025. PMID: 40266922 Free PMC article.
References
-
- Buchacher A, Iberer G. Purification of intravenous immunoglobulin G from human plasma – aspects of yield and virus safety. Biotechnol J. 2006;1:148–163. - PubMed
-
- Chang CE, Eo HG, Lee YS, Chung SK, Shin JS, Lah YK, Park CW, Jung JT, Huh JW, Lee SM. Human intravenous immunoglobulin preparation and virus inactivation by pasteurization and solvent detergent treatment. Prep Biochem Biotechnol. 2000;30:177–197. - PubMed
-
- Singh S, Miura K, Zhou H, Muratova O, Keegan B, Miles A, Martin LB, Saul AJ, Miller LH, Long CA. Immunity to recombinant Plasmodium falciparum merozoite surface protein 1 (MSP1): protection in Aotus nancymai monkeys strongly correlates with anti-MSP1 antibody titers and in vitro parasite-inhibitory activity. Infect Immun. 2006;74:4573–80. - PMC - PubMed
-
- Parkkinen J, Rahola A, von Bonsdorff L, Tolo H, Torma E. A modified caprylic acid method for manufacturing immunoglobulin G from human plasma with high yield and efficient virus clearance. Vox Sang. 2006;90:97–104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

