Exposing plasmids as the Achilles' heel of drug-resistant bacteria
- PMID: 18625335
- PMCID: PMC2570263
- DOI: 10.1016/j.cbpa.2008.06.015
Exposing plasmids as the Achilles' heel of drug-resistant bacteria
Abstract
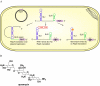
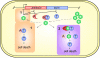
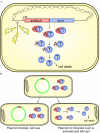
Many multidrug-resistant bacterial pathogens harbor large plasmids that encode proteins conferring resistance to antibiotics. Although the acquisition of these plasmids often enables bacteria to survive in the presence of antibiotics, it is possible that plasmids also represent a vulnerability that can be exploited in tailored antibacterial therapy. This review highlights three recently described strategies designed to specifically combat bacteria harboring such plasmids: inhibition of plasmid conjugation, inhibition of plasmid replication, and exploitation of plasmid-encoded toxin-antitoxin systems.
Figures



References
-
- Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, Bartlett JG, Edwards J., Jr. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155–164. - PubMed
-
- Alekshun MN, Levy SB. Molecular mechanisms of antibacterial multidrug resistance. Cell. 2007;128:1037–1050. - PubMed
-
- Wright GD. The antibiotic resistome: the nexus of chemical and genetic diversity. Nat Rev Microbiol. 2007;5:175–186. - PubMed
-
- Alanis AJ. Resistance to antibiotics: are we in the post-antibiotic era? Arch Med Res. 2005;36:697–705. - PubMed
-
- Projan SJ. Why is big Pharma getting out of antibacterial drug discovery? Curr Opin Microbiol. 2003;6:427–430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

