cAMP-dependent protein kinase A (PKA) signaling induces TNFR1 exosome-like vesicle release via anchoring of PKA regulatory subunit RIIbeta to BIG2
- PMID: 18625701
- PMCID: PMC2533074
- DOI: 10.1074/jbc.M804966200
cAMP-dependent protein kinase A (PKA) signaling induces TNFR1 exosome-like vesicle release via anchoring of PKA regulatory subunit RIIbeta to BIG2
Abstract
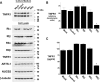
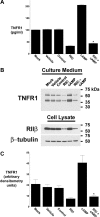
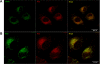
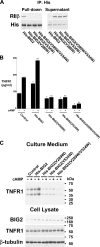
The 55-kDa TNFR1 (type I tumor necrosis factor receptor) can be released to the extracellular space by two mechanisms, the proteolytic cleavage and shedding of soluble receptor ectodomains and the release of full-length receptors within exosome-like vesicles. We have shown that the brefeldin A-inhibited guanine nucleotide exchange protein BIG2 associates with TNFR1 and selectively modulates the release of TNFR1 exosome-like vesicles via an ARF1- and ARF3-dependent mechanism. Here, we assessed the role of BIG2 A kinase-anchoring protein (AKAP) domains in the regulation of TNFR1 exosome-like vesicle release from human vascular endothelial cells. We show that 8-bromo-cyclic AMP induced the release of full-length, 55-kDa TNFR1 within exosome-like vesicles via a protein kinase A (PKA)-dependent mechanism. Using RNA interference to decrease specifically the levels of individual PKA regulatory subunits, we demonstrate that RIIbeta modulates both the constitutive and cAMP-induced release of TNFR1 exosome-like vesicles. Consistent with its AKAP function, BIG2 was required for the cAMP-induced PKA-dependent release of TNFR1 exosome-like vesicles via a mechanism that involved the binding of RIIbeta to BIG2 AKAP domains B and C. We conclude that both the constitutive and cAMP-induced release of TNFR1 exosome-like vesicles occur via PKA-dependent pathways that are regulated by the anchoring of RIIbeta to BIG2 via AKAP domains B and C. Thus, BIG2 regulates TNFR1 exosome-like vesicle release by two distinct mechanisms, as a guanine nucleotide exchange protein that activates class I ADP-ribosylation factors and as an AKAP for RIIbeta that localizes PKA signaling within cellular TNFR1 trafficking pathways.
Figures

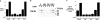

References
-
- Chen, G., and Goeddel, D. V. (2002) Science 296 1634-1635 - PubMed
-
- Locksley, R. M., Killeen, N., and Lenardo, M. J. (2001) Cell 104 487-501 - PubMed
-
- Wajant, H., Pfizenmaier, K., and Scheurich, P. (2003) Cell Death Differ. 10 45-65 - PubMed
-
- Wallach, D., Varfolomeev, E. E., Malinin, N. L., Goltsev, Y. V., Kovalenko, A. V., and Boldin, M. P. (1999) Annu. Rev. Immunol. 17 331-367 - PubMed
-
- Engelmann, H., Aderka, D., Rubinstein, M., Rotman, D., and Wallach, D. (1989) J. Biol. Chem. 264 11974-11980 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

