Characterization of complement factor H binding to Yersinia enterocolitica serotype O:3
- PMID: 18625735
- PMCID: PMC2519436
- DOI: 10.1128/IAI.00313-08
Characterization of complement factor H binding to Yersinia enterocolitica serotype O:3
Abstract
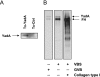
A number of bacteria bind factor H (FH), the negative regulator of the alternative complement pathway, to avoid complement-mediated killing. Here we show that a gram-negative enteric pathogen, Yersinia enterocolitica serotype O:3, uses two virulence-related outer membrane (OM) proteins to bind FH. With Y. enterocolitica O:3 mutant strains displaying different combinations of surface factors relevant to complement resistance, we demonstrated that the major receptor for FH is the OM protein YadA. Another OM protein, Ail, also contributes to FH binding provided that it is not blocked by distal parts of the lipopolysaccharide (i.e., the O antigen and the outer core hexasaccharide). Importantly, we demonstrated that surface-bound FH was functional; both YadA- and Ail-bound FH displayed cofactor activity for factor I-mediated cleavage of C3b. With truncated recombinant FH constructs, we located the binding site of Ail specifically to short consensus repeats 6 and 7 of FH, while YadA showed a novel type of FH-binding pattern and appears to bind FH throughout the entire FH molecule. We thus conclude that Y. enterocolitica, via YadA and Ail, recruits functionally active FH to its surface. FH binding appears to be an important mechanism of the complement resistance of this pathogen.
Figures



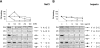
References
-
- al-Hendy, A., P. Toivanen, and M. Skurnik. 1991. The effect of growth temperature on the biosynthesis of Yersinia enterocolitica O:3 lipopolysaccharide: temperature regulates the transcription of the rfb but not of the rfa region. Microb. Pathog. 1081-86. - PubMed
-
- al-Hendy, A., P. Toivanen, and M. Skurnik. 1991. Expression cloning of Yersinia enterocolitica O:3 rfb gene cluster in Escherichia coli K12. Microb. Pathog. 1047-59. - PubMed
-
- Areschoug, T., M. Stalhammar-Carlemalm, I. Karlsson, and G. Lindahl. 2002. Streptococcal beta protein has separate binding sites for human factor H and IgA-Fc. J. Biol. Chem. 27712642-12648. - PubMed
-
- Barondess, J. J., and J. Beckwith. 1990. A bacterial virulence determinant encoded by lysogenic coliphage lambda. Nature 346871-874. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

