Characteristics of biofilm formation by Streptococcus mutans in the presence of saliva
- PMID: 18625741
- PMCID: PMC2519434
- DOI: 10.1128/IAI.00422-08
Characteristics of biofilm formation by Streptococcus mutans in the presence of saliva
Abstract
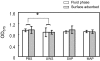
Interactions between salivary agglutinin and the adhesin P1 of Streptococcus mutans contribute to bacterial aggregation and mediate sucrose-independent adherence to tooth surfaces. We have examined biofilm formation by S. mutans UA159, and derivative strains carrying mutations affecting the localization or expression of P1, in the presence of fluid-phase or adsorbed saliva or salivary agglutinin preparations. Whole saliva- and salivary agglutinin-induced aggregation of S. mutans was adversely affected by the loss of P1 and sortase (SrtA) but not by the loss of trigger factor (RopA). Fluid-phase salivary agglutinin and, to a lesser extent, immobilized agglutinin inhibited biofilm development by S. mutans in the absence of sucrose, and whole saliva was more effective at decreasing biofilm formation than salivary agglutinin. Inhibition of biofilm development by salivary agglutinin was differently influenced by particular mutations, with the P1-deficient strain displaying a greater inhibition of biofilm development than the SrtA- or RopA-deficient strains. As expected, biofilm-forming capacities of all strains in the presence of salivary preparations were markedly enhanced in the presence of sucrose, although biofilm formation by the mutants was less efficient than that by the parental strain. Aeration strongly inhibited biofilm development, and the presence of salivary components did not restore biofilm formation in aerated conditions. The results disclose a potent ability of salivary constituents to moderate biofilm formation by S. mutans through P1-dependent and P1-independent pathways.
Figures







References
-
- Burne, R. A., R. G. Quivey, Jr., and R. E. Marquis. 1999. Physiologic homeostasis and stress responses in oral biofilms. Methods Enzymol. 310441-460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

