Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals
- PMID: 18625747
- PMCID: PMC2525604
- DOI: 10.1084/jem.20072683
Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals
Abstract
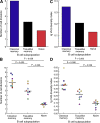
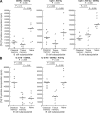
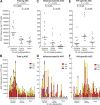
Human immunodeficiency virus (HIV) disease leads to impaired B cell and antibody responses through mechanisms that remain poorly defined. A unique memory B cell subpopulation (CD20(hi)/CD27(lo)/CD21(lo)) in human tonsillar tissues was recently defined by the expression of the inhibitory receptor Fc-receptor-like-4 (FCRL4). In this study, we describe a similar B cell subpopulation in the blood of HIV-viremic individuals. FCRL4 expression was increased on B cells of HIV-viremic compared with HIV-aviremic and HIV-negative individuals. It was enriched on B cells with a tissuelike memory phenotype (CD20(hi)/CD27(-)/CD21(lo)) when compared with B cells with a classical memory (CD27(+)) or naive (CD27(-)/CD21(hi)) B cell phenotype. Tissuelike memory B cells expressed patterns of homing and inhibitory receptors similar to those described for antigen-specific T cell exhaustion. The tissuelike memory B cells proliferated poorly in response to B cell stimuli, which is consistent with high-level expression of multiple inhibitory receptors. Immunoglobulin diversities and replication histories were lower in tissuelike, compared with classical, memory B cells, which is consistent with premature exhaustion. Strikingly, HIV-specific responses were enriched in these exhausted tissuelike memory B cells, whereas total immunoglobulin and influenza-specific responses were enriched in classical memory B cells. These data suggest that HIV-associated premature exhaustion of B cells may contribute to poor antibody responses against HIV in infected individuals.
Figures

References
-
- Giri, M.S., M. Nebozhyn, L. Showe, and L.J. Montaner. 2006. Microarray data on gene modulation by HIV-1 in immune cells: 2000-2006. J. Leukoc. Biol. 80:1031–1043. - PubMed
-
- Grossman, Z., M. Meier-Schellersheim, W.E. Paul, and L.J. Picker. 2006. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat. Med. 12:289–295. - PubMed
-
- De Milito, A. 2004. B lymphocyte dysfunctions in HIV infection. Curr. HIV Res. 2:11–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

