LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies
- PMID: 18626063
- PMCID: PMC2744109
- DOI: 10.1152/physrev.00033.2007
LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies
Abstract
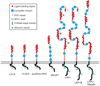
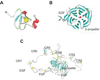
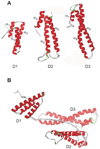
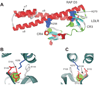
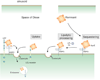
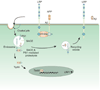
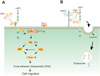
The LDL receptor-related protein (originally called LRP, but now referred to as LRP1) is a large endocytic receptor that is widely expressed in several tissues. LRP1 is a member of the LDL receptor family that plays diverse roles in various biological processes including lipoprotein metabolism, degradation of proteases, activation of lysosomal enzymes, and cellular entry of bacterial toxins and viruses. Deletion of the LRP1 gene leads to lethality in mice, revealing a critical, but as of yet, undefined role in development. Tissue-specific gene deletion studies reveal an important contribution of LRP1 in the vasculature, central nervous system, macrophages, and adipocytes. Three important properties of LRP1 dictate its diverse role in physiology: 1) its ability to recognize more than 30 distinct ligands, 2) its ability to bind a large number of cytoplasmic adaptor proteins via determinants located on its cytoplasmic domain in a phosphorylation-specific manner, and 3) its ability to associate with and modulate the activity of other transmembrane receptors such as integrins and receptor tyrosine kinases.
Figures
References
-
- Abe Ji, Deguchi J, Matsumoto T, Takuwa N, Noda M, Ohno M, Makuuchi M, Kurokawa K, Takuwa Y. Stimulated Activation of Platelet-Derived Growth Factor Receptor In Vivo in Balloon-Injured Arteries : A Link Between Angiotensin II and Intimal Thickening. Circulation. 1997;96:1906–1913. - PubMed
-
- Argraves KM, Battey FD, MacCalman CD, McCrae KR, Gåfvels M, Kozarsky KF, Chappell DA, Strauss JF, Strickland DK. The very low density lipoprotein receptor mediates the cellular catabolism of lipoprotein lipase and urokinase-plasminogen activator inhibitor type I complexes. J Biol Chem. 1995;270:26550–26557. - PubMed
-
- Baker RN, Cancilla PA, Pollock PS, Frommes SP. The movement of exogenous protein in experimental cerebral edema. An electron microscopic study after freeze-injury. J Neuropathol Exp Neurol. 1971;30:668–679. - PubMed
-
- Barker TH, Pallero MA, MacEwen MW, Tilden SG, Woods A, Murphy-Ullrich JE, Hagood JS. Thrombospondin-1-induced focal adhesion disassembly in fibroblasts requires Thy-1 surface expression, lipid raft integrity, and Src activation. J Biol Chem. 2004;279:23510–23516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL050784/HL/NHLBI NIH HHS/United States
- T32 HL007698/HL/NHLBI NIH HHS/United States
- P01 HL054710/HL/NHLBI NIH HHS/United States
- R01 HL072929/HL/NHLBI NIH HHS/United States
- T32 GM 07171/GM/NIGMS NIH HHS/United States
- T32 GM 08361-16/GM/NIGMS NIH HHS/United States
- HL 72929/HL/NHLBI NIH HHS/United States
- T32 HL 07698/HL/NHLBI NIH HHS/United States
- R01 HL079644/HL/NHLBI NIH HHS/United States
- HL 79644/HL/NHLBI NIH HHS/United States
- T32 GM007171/GM/NIGMS NIH HHS/United States
- HL 54710/HL/NHLBI NIH HHS/United States
- T32 GM008361/GM/NIGMS NIH HHS/United States
- HL 50784/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

