Dok-7 myasthenia: phenotypic and molecular genetic studies in 16 patients
- PMID: 18626973
- PMCID: PMC2570015
- DOI: 10.1002/ana.21408
Dok-7 myasthenia: phenotypic and molecular genetic studies in 16 patients
Abstract
Objective: Detailed analysis of phenotypic and molecular genetic aspects of Dok-7 myasthenia in 16 patients.
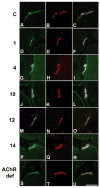
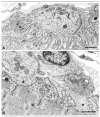
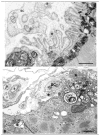
Methods: We assessed our patients by clinical and electromyographic studies, by intercostal muscle biopsies for in vitro microelectrode analysis of neuromuscular transmission and quantitative electron microscopy EM of 409 end plates (EPs), and by mutation analysis, and expression studies of the mutants.
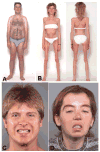
Results: The clinical spectrum varied from mild static limb-girdle weakness to severe generalized progressive disease. The synaptic contacts were single or multiple, and some, but not all, were small. In vitro microelectrode studies indicated variable decreases of the number of released quanta and of the synaptic response to acetylcholine; acetylcholine receptor (AChR) channel kinetics were normal. EM analysis demonstrated widespread and previously unrecognized destruction and remodeling of the EPs. Each patient carries 2 or more heteroallelic mutations: 11 in genomic DNA, 7 of which are novel; and 6 identifiable only in complementary DNA or cloned complementary DNA, 3 of which are novel. The pathogenicity of the mutations was confirmed by expression studies. Although the functions of Dok-7 include AChR beta-subunit phosphorylation and maintaining AChR site density, patient EPs showed normal AChR beta-subunit phosphorylation, and the AChR density on the remaining junctional folds appeared normal.
Interpretation: First, the clinical features of Dok-7 myasthenia are highly variable. Second, some mutations are complex and identifiable only in cloned complementary DNA. Third, Dok-7 is essential for maintaining not only the size but also the structural integrity of the EP. Fourth, the profound structural alterations at the EPs likely contribute importantly to the reduced safety margin of neuromuscular transmission.
Figures





References
-
- Engel AG, Sine SM. Current understanding of congenital myasthenic syndromes. Curr Opin Pharmacol. 2005;5:306–321. - PubMed
-
- Okada K, Inoue A, Okada M, et al. The muscle protein Dok-7 is essential for neuromuscular synaptogenesis. Science. 2006;312:1802–1805. - PubMed
-
- Beeson D, Higuchi O, Palace J, et al. Dok-7 mutations underlie a neuromuscular junction synaptopathy. Science. 2006;313:1975–1978. - PubMed
-
- Palace J, Lashley D, Newsom-Davis J, et al. Clinical features of the DOK7 neuromuscular junction synaptopathy. Brain. 2007;130:1507–1515. - PubMed
-
- Muller JS, Herczegfalvi A, Vilchez JJ, et al. Phenotypical spectrum of DOK7 mutations in congenital myasthenic syndromes. Brain. 2007;130:1497–1506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

