Chitosan cooperates with mesenchyme-derived factors in regulating salivary gland epithelial morphogenesis
- PMID: 18627424
- PMCID: PMC4498941
- DOI: 10.1111/j.1582-4934.2008.00425.x
Chitosan cooperates with mesenchyme-derived factors in regulating salivary gland epithelial morphogenesis
Abstract
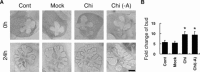
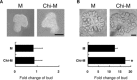
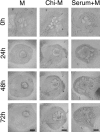
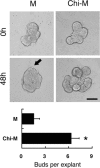
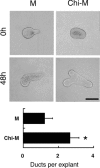
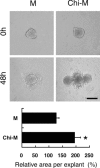
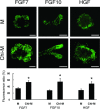
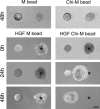
Chitosan is a widely used biocompatible biomaterial in the tissue regeneration, but its utility and application in the tissue morphogenesis of salivary gland remains unclear. The study aimed to explore the effects of chitosan on the epithelial morphogenesis of submandibular gland (SMG). With chitosan, the branching morphogenesis of the whole SMG explant was facilitated, and the morphogenetic-promoting effects of mesenchymal tissue on SMG were further enhanced. Furthermore, chitosan was competent to induce recombined SMG epithelium to form branches in the serum-free condition independently. In the presence of chitosan, the morphogenetic efficacy of mesenchyme-derived growth factors responsible for epithelial morphogenesis including fibroblast growth factors 7, fibroblast growth factor 10 and hepatocyte growth factor increased. The specific epithelial phenotype induced by individual growth factor, which was required for the accomplishment of salivary epithelial morphogenesis, was promoted by chitosan. Moreover, the proliferative and the chemotactic properties of these growth factors towards the SMG epithelia were also reinforced by chitosan. Therefore, in orchestrating and intensifying the essential mesenchyme-derived growth factors, chitosan is versatile in mediating SMG epithelium to form a predetermined phenotype more efficiently and comprehensively. This study suggested that chitosan is a morphogenetic-regulating biomaterial for salivary tissue, which might be useful for the future salivary gland investigation and regeneration.
Figures
Similar articles
-
Involvement of hepatocyte growth factor in branching morphogenesis of murine salivary gland.Dev Dyn. 2003 Oct;228(2):173-84. doi: 10.1002/dvdy.10377. Dev Dyn. 2003. PMID: 14517989
-
Connexin 43 Is Necessary for Salivary Gland Branching Morphogenesis and FGF10-induced ERK1/2 Phosphorylation.J Biol Chem. 2016 Jan 8;291(2):904-12. doi: 10.1074/jbc.M115.674663. Epub 2015 Nov 12. J Biol Chem. 2016. PMID: 26565022 Free PMC article.
-
Biomaterial mediated epithelial-mesenchymal interaction of salivary tissue under serum free condition.Biomaterials. 2010 Jan;31(2):288-95. doi: 10.1016/j.biomaterials.2009.09.052. Epub 2009 Oct 22. Biomaterials. 2010. PMID: 19853295
-
Branching morphogenesis in the fetal mouse submandibular gland is codependent on growth factors and extracellular matrix.J Med Invest. 2009;56 Suppl:228-33. doi: 10.2152/jmi.56.228. J Med Invest. 2009. PMID: 20224186 Review.
-
Regulatory Mechanisms Driving Salivary Gland Organogenesis.Curr Top Dev Biol. 2015;115:111-30. doi: 10.1016/bs.ctdb.2015.07.029. Epub 2015 Oct 21. Curr Top Dev Biol. 2015. PMID: 26589923 Free PMC article. Review.
Cited by
-
Bioengineering in salivary gland regeneration.J Biomed Sci. 2022 Jun 6;29(1):35. doi: 10.1186/s12929-022-00819-w. J Biomed Sci. 2022. PMID: 35668440 Free PMC article. Review.
-
Salivary Gland Bioengineering.Bioengineering (Basel). 2023 Dec 26;11(1):28. doi: 10.3390/bioengineering11010028. Bioengineering (Basel). 2023. PMID: 38247905 Free PMC article. Review.
-
Chitin-based materials in tissue engineering: applications in soft tissue and epithelial organ.Int J Mol Sci. 2011;12(3):1936-63. doi: 10.3390/ijms12031936. Epub 2011 Mar 17. Int J Mol Sci. 2011. PMID: 21673932 Free PMC article. Review.
-
Data supporting chitosan facilitates structure formation of the salivary gland by regulating the basement membrane components.Data Brief. 2015 Jul 17;4:551-8. doi: 10.1016/j.dib.2015.07.006. eCollection 2015 Sep. Data Brief. 2015. PMID: 26306324 Free PMC article.
-
Border patrol: insights into the unique role of perlecan/heparan sulfate proteoglycan 2 at cell and tissue borders.Matrix Biol. 2014 Feb;34:64-79. doi: 10.1016/j.matbio.2013.08.004. Epub 2013 Aug 31. Matrix Biol. 2014. PMID: 24001398 Free PMC article. Review.
References
-
- Vissink A, Jansma J, Spijkervet FK, et al. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:199–212. - PubMed
-
- Warde P, O’Sullivan B, Aslanidis J, et al. A phase III placebo-controlled trial of oral pilocarpine in patients undergoing radiotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;54:9–13. - PubMed
-
- Patel VN, Rebustini IT, Hoffman MP. Salivary gland branching morphogenesis. Differentiation. 2006;74:349–64. - PubMed
-
- Nogawa H, Takahashi Y. Substitution for mesenchyme by basement-membrane-like substratum and epidermal growth factor in inducing branching morphogenesis of mouse salivary epithelium. Development. 1991;112:855–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

