Chlamydial genes shed light on the evolution of photoautotrophic eukaryotes
- PMID: 18627593
- PMCID: PMC2490706
- DOI: 10.1186/1471-2148-8-203
Chlamydial genes shed light on the evolution of photoautotrophic eukaryotes
Abstract
Background: Chlamydiae are obligate intracellular bacteria of protists, invertebrates and vertebrates, but have not been found to date in photosynthetic eukaryotes (algae and embryophytes). Genes of putative chlamydial origin, however, are present in significant numbers in sequenced genomes of photosynthetic eukaryotes. It has been suggested that such genes were acquired by an ancient horizontal gene transfer from Chlamydiae to the ancestor of photosynthetic eukaryotes. To further test this hypothesis, an extensive search for proteins of chlamydial origin was performed using several recently sequenced algal genomes and EST databases, and the proteins subjected to phylogenetic analyses.
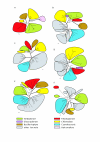
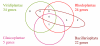
Results: A total of 39 proteins of chlamydial origin were retrieved from the photosynthetic eukaryotes analyzed and their identity verified through phylogenetic analyses. The distribution of the chlamydial proteins among four groups of photosynthetic eukaryotes (Viridiplantae, Rhodoplantae, Glaucoplantae, Bacillariophyta) was complex suggesting multiple acquisitions and losses. Evidence is presented that all except one of the chlamydial genes originated from an ancient endosymbiosis of a chlamydial bacterium into the ancestor of the Plantae before their divergence into Viridiplantae, Rhodoplantae and Glaucoplantae, i.e. more than 1.1 BYA. The chlamydial proteins subsequently spread through secondary plastid endosymbioses to other eukaryotes. Of 20 chlamydial proteins recovered from the genomes of two Bacillariophyta, 10 were of rhodoplant, and 10 of viridiplant origin suggesting that they were acquired by two different secondary endosymbioses. Phylogenetic analyses of concatenated sequences demonstrated that the viridiplant secondary endosymbiosis likely occurred before the divergence of Chlorophyta and Streptophyta.
Conclusion: We identified 39 proteins of chlamydial origin in photosynthetic eukaryotes signaling an ancient invasion of the ancestor of the Plantae by a chlamydial bacterium accompanied by horizontal gene transfer. Subsequently, chlamydial proteins spread through secondary endosymbioses to other eukaryotes. We conclude that intracellular chlamydiae likely persisted throughout the early history of the Plantae donating genes to their hosts that replaced their cyanobacterial/plastid homologs thus shaping early algal/plant evolution before they eventually vanished.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

