Cdc7-Drf1 kinase links chromosome cohesion to the initiation of DNA replication in Xenopus egg extracts
- PMID: 18628396
- PMCID: PMC2492736
- DOI: 10.1101/gad.1683308
Cdc7-Drf1 kinase links chromosome cohesion to the initiation of DNA replication in Xenopus egg extracts
Abstract
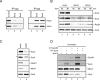
To establish functional cohesion between replicated sister chromatids, cohesin is recruited to chromatin before S phase. Cohesin is loaded onto chromosomes in the G1 phase by the Scc2-Scc4 complex, but little is known about how Scc2-Scc4 itself is recruited to chromatin. Using Xenopus egg extracts as a vertebrate model system, we showed previously that the chromatin association of Scc2 and cohesin is dependent on the prior establishment of prereplication complexes (pre-RCs) at origins of replication. Here, we report that Scc2-Scc4 exists in a stable complex with the Cdc7-Drf1 protein kinase (DDK), which is known to bind pre-RCs and activate them for DNA replication. Immunodepletion of DDK from Xenopus egg extracts impairs chromatin association of Scc2-Scc4, a defect that is reversed by wild-type, but not catalytically inactive DDK. A complex of Scc4 and the N terminus of Scc2 is sufficient for chromatin loading of Scc2-Scc4, but not for cohesin recruitment. These results show that DDK is required to tether Scc2-Scc4 to pre-RCs, and they underscore the intimate link between early steps in DNA replication and cohesion.
Figures
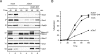




References
-
- Bailis J.M., Bernard P., Antonelli R., Allshire R.C., Forsburg S.L. Hsk1–Dfp1 is required for heterochromatin-mediated cohesion at centromeres. Nat. Cell Biol. 2003;5:1111–1116. - PubMed
-
- Bell S.P., Dutta A. DNA replication in eukaryotic cells. Annu. Rev. Biochem. 2002;71:333–374. - PubMed
-
- Bernard P., Drogat J., Maure J.F., Dheur S., Vaur S., Genier S., Javerzat J.P. A screen for cohesion mutants uncovers Ssl3, the fission yeast counterpart of the cohesin loading factor Scc4. Curr. Biol. 2006;16:875–881. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
