Irradiance-dependent photobleaching and pain in delta-aminolevulinic acid-photodynamic therapy of superficial basal cell carcinomas
- PMID: 18628462
- PMCID: PMC2810858
- DOI: 10.1158/1078-0432.CCR-07-5199
Irradiance-dependent photobleaching and pain in delta-aminolevulinic acid-photodynamic therapy of superficial basal cell carcinomas
Abstract
Purpose: In superficial basal cell carcinomas treated with photodynamic therapy with topical delta-aminolevulinic acid, we examined effects of light irradiance on photodynamic efficiency and pain. The rate of singlet-oxygen production depends on the product of irradiance and photosensitizer and oxygen concentrations. High irradiance and/or photosensitizer levels cause inefficient treatment from oxygen depletion in preclinical models.
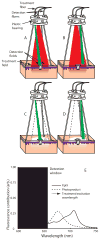
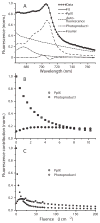
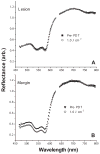
Experimental design: Self-sensitized photobleaching of protoporphyrin IX (PpIX) fluorescence was used as a surrogate metric for photodynamic dose. We developed instrumentation measuring fluorescence and reflectance from lesions and margins during treatment at 633 nm with various irradiances. When PpIX was 90% bleached, irradiance was increased to 150 mW/cm(2) until 200 J/cm(2) were delivered. Pain was monitored.
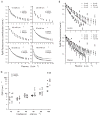
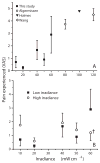
Results: In 33 superficial basal cell carcinomas in 26 patients, photobleaching efficiency decreased with increasing irradiance above 20 mW/cm(2), consistent with oxygen depletion. Fluences bleaching PpIX fluorescence 80% (D80) were 5.7 +/- 1.6, 4.5 +/- 0.3, 7.5 +/- 0.8, 7.4 +/- 0.3, 12.4 +/- 0.3, and 28.7 +/- 7.1 J/cm(2), respectively, at 10, 20, 40, 50, 60 and 150 mW/cm(2). At 20-150 mW/cm(2), D80 doses required 2.5-3.5 min; times for the total 200 J/cm(2) were 22.2-25.3 min. No significant pain occurred up to 50 mW/cm(2); pain was not significant when irradiance then increased. Clinical responses were comparable to continuous 150 mW/cm(2) treatment.
Conclusions: Photodynamic therapy with topical delta-aminolevulinic acid using approximately 40 mW/cm(2) at 633 nm is photodynamically efficient with minimum pain. Once PpIX is largely photobleached, higher irradiances allow efficient, rapid delivery of additional light. Optimal fluence at a single low irradiance is yet to be determined.
Figures
Similar articles
-
A retrospective review of pain control by a two-step irradiance schedule during topical ALA-photodynamic therapy of non-melanoma skin cancer.Lasers Surg Med. 2013 Feb;45(2):89-94. doi: 10.1002/lsm.22118. Epub 2013 Feb 6. Lasers Surg Med. 2013. PMID: 23390058 Free PMC article.
-
delta-Aminolevulinic acid and blue light photodynamic therapy for treatment of multiple basal cell carcinomas in two patients with nevoid basal cell carcinoma syndrome.Dermatol Surg. 2004 Jul;30(7):1054-61. doi: 10.1111/j.1524-4725.2004.30317.x. Dermatol Surg. 2004. PMID: 15209801 Review.
-
Photodynamic therapy of actinic keratosis at varying fluence rates: assessment of photobleaching, pain and primary clinical outcome.Br J Dermatol. 2004 Dec;151(6):1204-12. doi: 10.1111/j.1365-2133.2004.06211.x. Br J Dermatol. 2004. PMID: 15606516 Clinical Trial.
-
A prospective study of pain control by a 2-step irradiance schedule during topical photodynamic therapy of nonmelanoma skin cancer.Dermatol Surg. 2014 Dec;40(12):1390-4. doi: 10.1097/DSS.0000000000000183. Dermatol Surg. 2014. PMID: 25393353 Free PMC article.
-
Guidelines for topical photodynamic therapy: report of a workshop of the British Photodermatology Group.Br J Dermatol. 2002 Apr;146(4):552-67. doi: 10.1046/j.1365-2133.2002.04719.x. Br J Dermatol. 2002. PMID: 11966684 Review.
Cited by
-
Daylight-PDT: everything under the sun.Biochem Soc Trans. 2022 Apr 29;50(2):975-985. doi: 10.1042/BST20200822. Biochem Soc Trans. 2022. PMID: 35385082 Free PMC article. Review.
-
Two-step photodynamic therapy for facial acne: a randomized controlled trial of pain reduction with 630 nm red light laser.Lasers Med Sci. 2025 Apr 7;40(1):178. doi: 10.1007/s10103-025-04437-4. Lasers Med Sci. 2025. PMID: 40192834 Clinical Trial.
-
Susceptibility of bacterial species commonly found in abdominal abscesses to low-dose photodynamic therapy: Effects of methylene blue concentration, fluence rate, and fluence.Photochem Photobiol. 2025 Mar 26:10.1111/php.14092. doi: 10.1111/php.14092. Online ahead of print. Photochem Photobiol. 2025. PMID: 40135394
-
A retrospective review of pain control by a two-step irradiance schedule during topical ALA-photodynamic therapy of non-melanoma skin cancer.Lasers Surg Med. 2013 Feb;45(2):89-94. doi: 10.1002/lsm.22118. Epub 2013 Feb 6. Lasers Surg Med. 2013. PMID: 23390058 Free PMC article.
-
Recovery of intrinsic fluorescence from single-point interstitial measurements for quantification of doxorubicin concentration.Lasers Surg Med. 2013 Oct;45(8):542-50. doi: 10.1002/lsm.22166. Epub 2013 Aug 23. Lasers Surg Med. 2013. PMID: 24037853 Free PMC article.
References
-
- Zeitouni NC, Oseroff AR, Shieh S. Photodynamic therapy for nonmelanoma skin cancers. Current review and update Mol Immunol. 2003;39:1133–6. - PubMed
-
- Foster TH, Murant RS, Bryant RG, Knox RS, Gibson SL, Hilf R. Oxygen consumption and diffusion effects in photodynamic therapy. Radiat Res. 1991;126:296–303. - PubMed
-
- Sitnik TM, Henderson BW. The effect of fluence rate on tumor and normal tissue responses to photodynamic therapy. Photochem Photobiol. 1998;67:462–6. - PubMed
-
- Robinson DJ, de Bruijn HS, van d V, Stringer MR, Brown SB, Star WM. Fluorescence photobleaching of ALA-induced protoporphyrin IX during photodynamic therapy of normal hairless mouse skin: the effect of light dose and irradiance and the resulting biological effect. Photochem Photobiol. 1998;67:140–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

