Incretin-based therapies in type 2 diabetes mellitus
- PMID: 18628530
- PMCID: PMC2579648
- DOI: 10.1210/jc.2007-2109
Incretin-based therapies in type 2 diabetes mellitus
Abstract
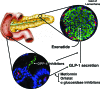
Context: Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide are incretins secreted from enteroendocrine cells postprandially in part to regulate glucose homeostasis. Dysregulation of these hormones is evident in type 2 diabetes mellitus (T2DM). Two new drugs, exenatide (GLP-1 mimetic) and sitagliptin [dipeptidyl peptidase (DPP) 4 inhibitor], have been approved by regulatory agencies for treating T2DM. Liraglutide (GLP-1 mimetic) and vildagliptin (DPP 4 inhibitor) are expected to arrive on the market soon.
Evidence acquisition: The background of incretin-based therapy and selected clinical trials of these four drugs are reviewed. A MEDLINE search was conducted for published articles using the key words incretin, glucose-dependent insulinotropic polypeptide, GLP-1, exendin-4, exenatide, DPP 4, liraglutide, sitagliptin, and vildagliptin.
Evidence synthesis: Exenatide and liraglutide are injection based. Three-year follow-up data on exenatide showed a sustained weight loss and glycosylated hemoglobin (HbA(1c)) reduction of 1%. Nausea and vomiting are common. Results from phase 3 studies are pending on liraglutide. Sitagliptin and vildagliptin are orally active. In 24-wk studies, sitagliptin reduces HbA(1c) by 0.6-0.8% as monotherapy, 1.8% as initial combination therapy with metformin, and 0.7% as add-on therapy to metformin. Vildagliptin monotherapy lowered HbA(1c) by 1.0-1.4% after 24 wk. Their major side effects are urinary tract and nasopharyngeal infections and headaches. Exenatide and liraglutide cause weight loss, whereas sitagliptin and vildagliptin do not.
Conclusions: The availability of GLP-1 mimetics and DPP 4 inhibitors has increased our armamentarium for treating T2DM. Unresolved issues such as the effects of GLP-1 mimetics and DPP 4 inhibitors on beta-cell mass, the mechanism by which GLP-1 mimetics lowers glucagon levels, and exactly how DPP 4 inhibitors lead to a decline in plasma glucose levels without an increase in insulin secretion, need further research.
Figures

References
-
- Elrick H, Stimmler L, Hlad Jr CJ, Arai Y 1964 Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab 24:1076–1082 - PubMed
-
- McIntyre N, Holdsworth CD, Turner DS 1965 Intestinal factors in the control of insulin secretion. J Clin Endocrinol Metab 25:1317–1324 - PubMed
-
- Theodorakis MJ, Carlson O, Michopoulos S, Doyle ME, Juhaszova M, Petraki K, Egan JM 2006 Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab 290:E550–E559 - PubMed
-
- Vollmer K, Holst JJ, Baller B, Ellrichmann M, Nauck MA, Schmidt WE, Meier JJ 2008 Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes 57:678–687 - PubMed
-
- Vilsboll T, Krarup T, Deacon CF, Madsbad S, Holst JJ 2001 Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes 50:609–613 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

