Genomic profiles of damage and protection in human intracerebral hemorrhage
- PMID: 18628781
- PMCID: PMC2745827
- DOI: 10.1038/jcbfm.2008.77
Genomic profiles of damage and protection in human intracerebral hemorrhage
Abstract
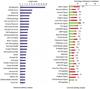
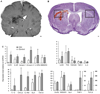
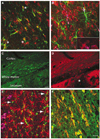
Intracerebral hemorrhage (ICH) produces a high rate of death and disability. The molecular mechanisms of damage in perihematomal tissue in humans have not been systematically characterized. This study determines the gene expression profile and molecular networks that are induced in human perihematomal tissue through molecular analysis of tissue obtained from endoscopic clot evacuation. Differentially expressed genes and their cellular origin were confirmed in a mouse model of ICH. A total of 624 genes showed altered regulation in human ICH. Bioinformatic analysis shows that these genes form interconnected networks of proinflammatory, anti-inflammatory, and neuronal signaling cascades. Intracerebral hemorrhage evokes coordinated upregulation of proinflammatory signaling through specific cytokines and chemokines and their downstream molecular pathways. Anti-inflammatory networks are also induced by ICH, including annexins A1 and A2 and transforming growth factor beta (TGFbeta) and their intracellular cascades. Intracerebral hemorrhage downregulates many neuronal signaling systems, including the N-methyl-D-aspartic acid (NMDA) receptor complex and membrane ion channels. Select portions of these molecular networks were confirmed in the mouse, and the proteins in a subset of these networks localized to subsets of neurons, oligodendrocytes, or leukocytes. These inflammatory and anti-inflammatory networks interact at several key points in neutrophil signaling, apoptotic cell death, and protease responses, and indicate that secondary damage in ICH activates opposing molecular systems.
Conflict of interest statement
The authors state no conflict of interest.
Figures
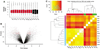


References
-
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD–95 protein interactions. Science. 2002;298:846–850. - PubMed
-
- Ardizzone TD, Lu A, Wagner KR, Tang Y, Ran R, Sharp FR. Glutamate receptor blockade attenuates glucose hypermetabolism in perihematomal brain after experimental intracerebral hemorrhage in rat. Stroke. 2004;35:2587–2591. - PubMed
-
- Bajetto A, Bonavia R, Barbero S, Pirani P, Florio T, Schettini G. Chemokines and their receptors in the central nervous system. Front Neuroendocrinol. 2001;22:147–184. - PubMed
-
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

