Autoimmunity in the immune privileged eye: pathogenic and regulatory T cells
- PMID: 18629448
- PMCID: PMC2756228
- DOI: 10.1007/s12026-008-8031-3
Autoimmunity in the immune privileged eye: pathogenic and regulatory T cells
Abstract
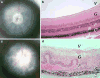
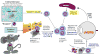
Experimental autoimmune uveitis (EAU) in animals serves as a model of human uveitis. EAU can be induced in mice by immunization with the retinal antigen interphotoreceptor retinoid binding protein (IRBP) in complete Freund's adjuvant (CFA) or by IRBP-pulsed mature dendritic cells, and can be driven either by a Th17 or a Th1 effector response, depending on the model. The direction of the response is affected by conditions present during the exposure to antigen, including the quality/quantity of innate receptor stimulation and/or type of APC. IL-17 and IFN-gamma production by innate cells such as NKT may also affect the disease process. If exposure to antigen is via a hydrodynamic DNA vaccination with an IRBP-encoding plasmid, the response is directed to a regulatory phenotype, and disease is ameliorated or prevented. Our data shed light on effector and regulatory responses in autoimmune disease, provide balance to the Th1/Th17 paradigm and help to explain the clinical heterogeneity of human uveitis, which occurs in the face of responses to the same ocular antigen(s).
Figures

References
-
- Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491–500. discussion 500. - PubMed
-
- Nussenblatt RB, Whitcup SM. Uveitis: fundamentals and clinical practice. 3. Philadelphia, PA: Mosby (Elsevier); 2004.
-
- Gery I, Nussenblatt RB, Chan CC, Caspi RR. The molecular pathology of autoimmune diseases. 2. New York, NY: Taylor and Francis; 2002. Autoimmune diseases of the eye; pp. 978–98.
-
- Agarwal RK, Caspi RR. Rodent models of experimental autoimmune uveitis. Methods Mol Med. 2004;102:395–419. - PubMed
-
- Stein-Streilein J, Streilein JW. Anterior chamber associated immune deviation (ACAID): regulation, biological relevance, and implications for therapy. Int Rev Immunol. 2002;21:123–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

