Morphogenesis of the node and notochord: the cellular basis for the establishment and maintenance of left-right asymmetry in the mouse
- PMID: 18629866
- PMCID: PMC2593123
- DOI: 10.1002/dvdy.21598
Morphogenesis of the node and notochord: the cellular basis for the establishment and maintenance of left-right asymmetry in the mouse
Abstract
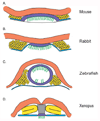
Establishment of left-right asymmetry in the mouse embryo depends on leftward laminar fluid flow in the node, which initiates a signaling cascade that is confined to the left side of the embryo. Leftward fluid flow depends on two cellular processes: motility of the cilia that generate the flow and morphogenesis of the node, the structure where the cilia reside. Here, we provide an overview of the current understanding and unresolved questions about the regulation of ciliary motility and node structure. Analysis of mouse mutants has shown that the motile cilia must have a specific structure and length, and that they must point posteriorly to generate the necessary leftward fluid flow. However, the precise structure of the motile cilia is not clear and the mechanisms that position cilia on node cells have not been defined. The mouse node is a teardrop-shaped pit at the distal tip of the early embryo, but the morphogenetic events that create the mature node from cells derived from the primitive streak are only beginning to be characterized. Recent live imaging experiments support earlier scanning electron microscopy (SEM) studies and show that node assembly is a multi-step process in which clusters of node precursors appear on the embryo surface as overlying endoderm cells are removed. We present additional SEM and confocal microscopy studies that help define the transition stages during node morphogenesis. After the initiation of left-sided signaling, the notochordal plate, which is contiguous with the node, generates a barrier at the embryonic midline that restricts the cascade of gene expression to the left side of the embryo. The field is now poised to dissect the genetic and cellular mechanisms that create and organize the specialized cells of the node and midline that are essential for left-right asymmetry.
(c) 2008 Wiley-Liss, Inc.
Figures






References
-
- Abdelkhalek HB, Beckers A, Schuster-Gossler K, Pavlova MN, Burkhardt H, Lickert H, Rossant J, Reinhardt R, Schalkwyk LC, Muller I, Herrmann BG, Ceolin M, Rivera-Pomar R, Gossler A. The mouse homeobox gene Not is required for caudal notochord development and affected by the truncate mutation. Genes Dev. 2004;18:1725–1736. - PMC - PubMed
-
- Amack JD, Wang X, Yost HJ. Two T-box genes play independent and cooperative roles to regulate morphogenesis of ciliated Kupffer's vesicle in zebrafish. Dev Biol. 2007;310:196–210. - PubMed
-
- Amack JD, Yost HJ. The T box transcription factor no tail in ciliated cells controls zebrafish left-right asymmetry. Curr Biol. 2004;14:685–690. - PubMed
-
- Ang SL, Jin O, Rhinn M, Daigle N, Stevenson L, Rossant J. A targeted mouse Otx2 mutation leads to severe defects in gastrulation and formation of axial mesoderm and to deletion of rostral brain. Development. 1996;122:243–252. - PubMed
-
- Ang SL, Rossant J. HNF-3 beta is essential for node and notochord formation in mouse development. Cell. 1994;78:561–574. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

