Slow self-activation enhances the potency of viridin prodrugs
- PMID: 18630894
- PMCID: PMC2663427
- DOI: 10.1021/jm800374f
Slow self-activation enhances the potency of viridin prodrugs
Abstract
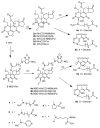
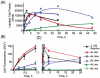
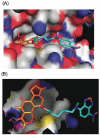
When the viridin wortmannin (Wm) is modified by reaction with certain nucleophiles at the C20 position, the compounds obtained exhibit an improved antiproliferative activity even though a covalent reaction between C20 and a lysine in the active site of PI3 kinase is essential to Wm's ability to inhibit this enzyme. Here we show that this improved potency results from an intramolecular attack by the C6 hydroxyl group that slowly converts these inactive prodrugs to the active species Wm over the 48 h duration of the antiproliferative assay. Our results provide a guide for selecting Wm-like compounds to maximize kinase inhibition with the variety of protocols used to assess the role of PI3 kinase in biological systems, or for achieving optimal therapeutic effects in vivo . In addition, the slow self-activation of WmC20 derivatives provides a mechanism that can be exploited to obtain kinase inhibitors endowed with physical and pharmacokinetic properties far different from man-made kinase inhibitors because they do not bind to kinase active sites.
Figures


References
-
- Wipf P, Halter Robert J. Chemistry and biology of wortmannin. Organic & biomolecular chemistry. 2005;3:2053–61. - PubMed
-
- Powis G, Bonjouklian R, Berggren MM, Gallegos A, Abraham R, Ashendel C, Zalkow L, Matter WF, Dodge J, Grindey G, et al. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994;54:2419–23. - PubMed
-
- Creemer LC, Kirst HA, Vlahos CJ, Schultz RM. Synthesis and in vitro evaluation of new wortmannin esters: potent inhibitors of phosphatidylinositol 3-kinase. J Med Chem. 1996;39:5021–4. - PubMed
-
- Liu Y, Shreder KR, Gai W, Corral S, Ferris DK, Rosenblum JS. Wortmannin, a widely used phosphoinositide 3-kinase inhibitor, also potently inhibits mammalian polo-like kinase. Chem Biol. 2005;12:99–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information

