Selective nucleoside triphosphate diphosphohydrolase-2 (NTPDase2) inhibitors: nucleotide mimetics derived from uridine-5'-carboxamide
- PMID: 18630897
- PMCID: PMC5241159
- DOI: 10.1021/jm800175e
Selective nucleoside triphosphate diphosphohydrolase-2 (NTPDase2) inhibitors: nucleotide mimetics derived from uridine-5'-carboxamide
Abstract
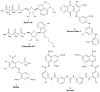
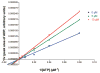
Ecto-nucleoside triphosphate diphosphohydrolases (E-NTPDases, subtypes 1, 2, 3, 8 of NTPDases) dephosphorylate nucleoside tri- and diphosphates to the corresponding di- and monophosphates. In the present study we synthesized adenine and uracil nucleotide mimetics, in which the phosphate residues were replaced by phosphonic acid esters attached to the nucleoside at the 5'-position by amide linkers. Among the synthesized uridine derivatives, we identified the first potent and selective inhibitors of human NTPDase2. The most potent compound was 19a (PSB-6426), which was a competitive inhibitor of NTPDase2 exhibiting a K i value of 8.2 microM and selectivity versus other NTPDases. It was inactive toward uracil nucleotide-activated P2Y 2, P2Y 4, and P2Y 6 receptor subtypes. Compound 19a was chemically and metabolically highly stable. In contrast to the few known (unselective) NTPDase inhibitors, 19a is an uncharged molecule and may be perorally bioavailable. NTPDase2 inhibitors have potential as novel cardioprotective drugs for the treatment of stroke and for cancer therapy.
Figures

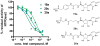
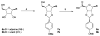
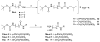

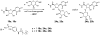
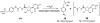
Similar articles
-
Development of Anthraquinone Derivatives as Ectonucleoside Triphosphate Diphosphohydrolase (NTPDase) Inhibitors With Selectivity for NTPDase2 and NTPDase3.Front Pharmacol. 2020 Aug 27;11:1282. doi: 10.3389/fphar.2020.01282. eCollection 2020. Front Pharmacol. 2020. PMID: 32973513 Free PMC article.
-
Synthesis, In-vitro evaluation and molecular docking studies of oxoindolin phenylhydrazine carboxamides as potent and selective inhibitors of ectonucleoside triphosphate diphosphohydrolase (NTPDase).Bioorg Chem. 2021 Jul;112:104957. doi: 10.1016/j.bioorg.2021.104957. Epub 2021 Apr 30. Bioorg Chem. 2021. PMID: 34020240
-
Highly Potent and Selective Ectonucleoside Triphosphate Diphosphohydrolase (ENTPDase1, 2, 3 and 8) Inhibitors Having 2-substituted-7- trifluoromethyl-thiadiazolopyrimidones Scaffold.Med Chem. 2020;16(5):689-702. doi: 10.2174/1573406415666190614095821. Med Chem. 2020. PMID: 31203806
-
Ecto-nucleotidase inhibitors: recent developments in drug discovery.Mini Rev Med Chem. 2015;15(1):21-33. doi: 10.2174/1389557515666150219115141. Mini Rev Med Chem. 2015. PMID: 25694081 Review.
-
Therapeutic potentials of ecto-nucleoside triphosphate diphosphohydrolase, ecto-nucleotide pyrophosphatase/phosphodiesterase, ecto-5'-nucleotidase, and alkaline phosphatase inhibitors.Med Res Rev. 2014 Jul;34(4):703-43. doi: 10.1002/med.21302. Epub 2013 Sep 23. Med Res Rev. 2014. PMID: 24115166 Review.
Cited by
-
Epitope mapping in cell surface proteins by site-directed masking: defining the structural elements of NTPDase3 inhibition by a monoclonal antibody.Protein Eng Des Sel. 2010 Jul;23(7):579-88. doi: 10.1093/protein/gzq027. Epub 2010 May 27. Protein Eng Des Sel. 2010. PMID: 20511214 Free PMC article.
-
Ectonucleotidases in the kidney.Purinergic Signal. 2009 Dec;5(4):501-11. doi: 10.1007/s11302-009-9152-4. Epub 2009 Mar 31. Purinergic Signal. 2009. PMID: 19333785 Free PMC article.
-
Dependence of Leishmania parasite on host derived ATP: an overview of extracellular nucleotide metabolism in parasite.J Parasit Dis. 2019 Mar;43(1):1-13. doi: 10.1007/s12639-018-1061-4. Epub 2018 Dec 1. J Parasit Dis. 2019. PMID: 30956439 Free PMC article. Review.
-
Inhibition of ecto-ATPase activity by curcumin in hepatocellular carcinoma HepG2 cells.J Physiol Sci. 2012 Jan;62(1):53-8. doi: 10.1007/s12576-011-0176-5. Epub 2011 Sep 20. J Physiol Sci. 2012. PMID: 21932081 Free PMC article.
-
Parallel solution-phase synthesis and general biological activity of a uridine antibiotic analog library.ACS Comb Sci. 2014 May 12;16(5):232-7. doi: 10.1021/co4001452. Epub 2014 Apr 3. ACS Comb Sci. 2014. PMID: 24661222 Free PMC article.
References
-
- Abbracchio MP, Burnstock G, Boeynaems J, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. - PMC - PubMed
-
- Zimmermann H. Ectonucleotidases: some recent developments and a note on nomenclature. Drug Dev Res. 2001;52:44–56.
-
- Stefan C, Jansen S, Bollen M. NPP-type ectophosphodiesterases: unity in diversity. Trends Biochem Sci. 2005;30:542–550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases

