Two essential MYST-family proteins display distinct roles in histone H4K10 acetylation and telomeric silencing in trypanosomes
- PMID: 18631159
- PMCID: PMC2556858
- DOI: 10.1111/j.1365-2958.2008.06346.x
Two essential MYST-family proteins display distinct roles in histone H4K10 acetylation and telomeric silencing in trypanosomes
Abstract
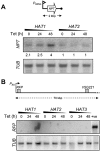
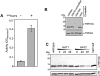
Chromatin modification is important for virtually all aspects of DNA metabolism but little is known about the consequences of such modification in trypanosomatids, early branching protozoa of significant medical and veterinary importance. MYST-family histone acetyltransferases in other species function in transcription regulation, DNA replication, recombination and repair. Trypanosoma brucei HAT3 was recently shown to acetylate histone H4K4 and we now report characterization of all three T. brucei MYST acetyltransferases (HAT1-3). First, GFP-tagged HAT1-3 all localize to the trypanosome nucleus. While HAT3 is dispensable, both HAT1 and HAT2 are essential for growth. Strains with HAT1 knock-down display mitosis without nuclear DNA replication and also specific de-repression of a telomeric reporter gene, a rare example of transcription control in an organism with widespread and constitutive polycistronic transcription. Finally, we show that HAT2 is responsible for H4K10 acetylation. By analogy to the situation in Saccharomyces cerevisiae, we discuss low-level redundancy of acetyltransferase function in T. brucei and suggest that two MYST-family acetyltransferases are essential due to the absence of a Gcn5 homologue. The results are also consistent with the idea that HAT1 contributes to establishing boundaries between transcriptionally active and repressed telomeric domains in T. brucei.
Figures




References
-
- Akhtar A, Zink D, Becker PB. Chromodomains are protein–RNA interaction modules. Nature. 2000;407:405–409. - PubMed
-
- Alsford S, Kawahara T, Isamah C, Horn D. A sirtuin in the African trypanosome is involved in both DNA repair and telomeric gene silencing but is not required for antigenic variation. Mol Microbiol. 2007;63:724–736. - PubMed
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York, NY: John Wiley and Sons; 1998.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

