PKA and ERK, but not PKC, in the amygdala contribute to pain-related synaptic plasticity and behavior
- PMID: 18631385
- PMCID: PMC2490682
- DOI: 10.1186/1744-8069-4-26
PKA and ERK, but not PKC, in the amygdala contribute to pain-related synaptic plasticity and behavior
Abstract
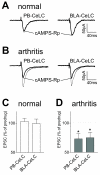
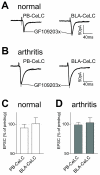
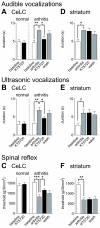
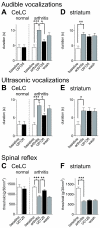
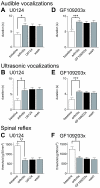
The laterocapsular division of the central nucleus of the amygdala (CeLC) has emerged as an important site of pain-related plasticity and pain modulation. Glutamate and neuropeptide receptors in the CeLC contribute to synaptic and behavioral changes in the arthritis pain model, but the intracellular signaling pathways remain to be determined. This study addressed the role of PKA, PKC, and ERK in the CeLC. Adult male Sprague-Dawley rats were used in all experiments. Whole-cell patch-clamp recordings of CeLC neurons were made in brain slices from normal rats and from rats with a kaolin/carrageenan-induced monoarthritis in the knee (6 h postinduction). Membrane-permeable inhibitors of PKA (KT5720, 1 microM; cAMPS-Rp, 10 microM) and ERK (U0126, 1 microM) activation inhibited synaptic plasticity in slices from arthritic rats but had no effect on normal transmission in control slices. A PKC inhibitor (GF109203x, 1 microM) and an inactive structural analogue of U0126 (U0124, 1 microM) had no effect. The NMDA receptor-mediated synaptic component was inhibited by KT5720 or U0126; their combined application had additive effects. U0126 did not inhibit synaptic facilitation by forskolin-induced PKA-activation. Administration of KT5720 (100 microM, concentration in microdialysis probe) or U0126 (100 microM) into the CeLC, but not striatum (placement control), inhibited audible and ultrasonic vocalizations and spinal reflexes of arthritic rats but had no effect in normal animals. GF109203x (100 microM) and U0124 (100 microM) did not affect pain behavior. The data suggest that in the amygdala PKA and ERK, but not PKC, contribute to pain-related synaptic facilitation and behavior by increasing NMDA receptor function through independent signaling pathways.
Figures






References
-
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10:221–234. - PubMed
-
- Ikeda R, Takahashi Y, Inoue K, Kato F. NMDA receptor-independent synaptic plasticity in the central amygdala in the rat model of neuropathic pain. Pain. 2007;127:161–172. - PubMed
-
- Pedersen LH, Scheel-Kruger J, Blackburn-Munro G. Amygdala GABA-A receptor involvement in mediating sensory-discriminative and affective-motivational pain responses in a rat model of peripheral nerve injury. Pain. 2007;127:17–26. - PubMed
-
- Heinricher MM, McGaraughty S. Pain-modulating neurons and behavioral state. In: Lydic R and Baghdoyan HA, editor. Handbook of Behavioral State Control. New York, CRC Press; 1999. pp. 487–503.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

