Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein
- PMID: 18631454
- PMCID: PMC2704496
- DOI: 10.1016/j.immuni.2008.05.013
Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein
Abstract
The mammalian immune system discriminates between modes of cell death; necrosis often results in inflammation and adaptive immunity, whereas apoptosis tends to be anti-inflammatory and promote immune tolerance. We have examined apoptosis for the features responsible for tolerance; specifically, we looked at the roles of caspases and mitochondria. Our results show that caspase activation targeted the mitochondria to produce reactive oxygen species (ROS), which were critical to tolerance induction by apoptotic cells. ROS oxidized the potential danger signal high-mobility group box-1 protein (HMGB1) released from dying cells and thereby neutralized its stimulatory activity. Apoptotic cells failed to induce tolerance and instead stimulated immune responses by scavenging or by mutating a mitochondrial caspase target protein when ROS activity was prohibited. Similarly, blocking sites of oxidation in HMGB1 prevented tolerance induction by apoptotic cells. These results suggest that caspase-orchestrated mitochondrial events determine the impact of apoptotic cells on the immune response.
Figures



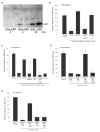
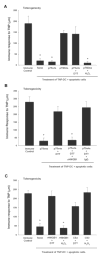

Comment in
-
ROS eliminate danger.Immunity. 2008 Jul 18;29(1):1-2. doi: 10.1016/j.immuni.2008.06.006. Immunity. 2008. PMID: 18631448
References
-
- Albert ML, Jegathesan M, Darnell RB. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat Immunol. 2001;2:1010–1017. - PubMed
-
- Andersson U, Erlandsson-Harris H, Yang H, Tracey KJ. HMGB1 as a DNA-binding cytokine. J Leukoc Biol. 2002;72:1084–1091. - PubMed
-
- Battisto JR, Bloom BR. Dual immunological unresponsiveness induced by cell membrane coupled hapten or antigen. Nature. 1966;212:156–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

