The biophysical function of pulmonary surfactant
- PMID: 18632313
- PMCID: PMC2669693
- DOI: 10.1016/j.resp.2008.05.018
The biophysical function of pulmonary surfactant
Abstract
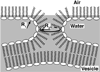
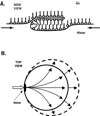
Pulmonary surfactant lowers surface tension in the lungs. Physiological studies indicate two key aspects of this function: that the surfactant film forms rapidly; and that when compressed by the shrinking alveolar area during exhalation, the film reduces surface tension to very low values. These observations suggest that surfactant vesicles adsorb quickly, and that during compression, the adsorbed film resists the tendency to collapse from the interface to form a 3D bulk phase. Available evidence suggests that adsorption occurs by way of a rate-limiting structure that bridges the gap between the vesicle and the interface, and that the adsorbed film avoids collapse by undergoing a process of solidification. Current models, although incomplete, suggest mechanisms that would partially explain both rapid adsorption and resistance to collapse as well as how different constituents of pulmonary surfactant might affect its behavior.
Figures





References
-
- Adamson AW, Gast AP. Physical Chemistry of Surfaces. New York: Wiley; 1997a. p. 4.
-
- Adamson AW, Gast AP. Physical Chemistry of Surfaces. New York: Wiley; 1997b. pp. 71–74.
-
- Allen SM, Thomas EL. The Structure of Materials. J. New York: Wiley; 1999. p. 31.
-
- Amrein M, von Nahmen A, Sieber M. A scanning force and fluorescence light microscopy study of the structure and function of a model pulmonary surfactant. Eur. Biophys. J. Biophys. Lett. 1997;26:349–357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

