Single treatment with RNAi against prion protein rescues early neuronal dysfunction and prolongs survival in mice with prion disease
- PMID: 18632556
- PMCID: PMC2474561
- DOI: 10.1073/pnas.0802759105
Single treatment with RNAi against prion protein rescues early neuronal dysfunction and prolongs survival in mice with prion disease
Abstract
Prion diseases are fatal neurodegenerative conditions for which there is no effective treatment. Prion propagation involves the conversion of cellular prion protein, PrP(C), to its conformational isomer, PrP(Sc), which accumulates in disease. Here, we show effective therapeutic knockdown of PrP(C) expression using RNAi in mice with established prion disease. A single administration of lentivirus expressing a shRNA targeting PrP into each hippocampus of mice with established prion disease significantly prolonged survival time. Treated animals lived 19% and 24% longer than mice given an "empty" lentivirus, or not treated, respectively. Lentivirally mediated RNAi of PrP also prevented the onset of behavioral deficits associated with early prion disease, reduced spongiform degeneration, and protected against neuronal loss. In contrast, mice receiving empty virus or no treatment developed early cognitive impairment and showed severe spongiosis and neuronal loss. The focal use of RNAi therapeutically in prion disease further supports strategies depleting PrP(C), which we previously established to be a valid target for prion-based treatments. This approach can now be used to define the temporal, quantitative, and regional requirements for PrP knockdown for effective treatment of prion disease and to explore mechanisms involved in predegenerative neuronal dysfunction and its rescue.
Conflict of interest statement
Conflict of interest statement: J.C. is a director and shareholder of D-Gen Limited, an academic spin-out company working in the field of prion disease diagnosis, decontamination, and therapeutics. D-Gen markets the ICSM35 and ICS18 antibodies used in this study.
Figures
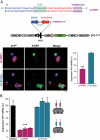
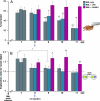
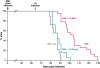
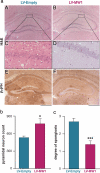

References
-
- Bueler H, et al. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. - PubMed
-
- Sailer A, et al. No propagation of prions in mice devoid of PrP. Cell. 1994;77:967–968. - PubMed
-
- Manson JC, et al. PrP gene dosage determines the timing but not the final intensity or distribution of lesions in scrapie pathology. Neurodegeneration. 1994;3:331–340. - PubMed
-
- Mallucci G, et al. Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science. 2003;302:871–874. - PubMed
-
- Mallucci GR, et al. Targeting cellular prion protein reverses early cognitive deficits and neurophysiological dysfunction in prion-infected mice. Neuron. 2007;53:325–335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

