Vimentin filaments support extension of tubulin-based microtentacles in detached breast tumor cells
- PMID: 18632620
- PMCID: PMC2859318
- DOI: 10.1158/0008-5472.CAN-07-6589
Vimentin filaments support extension of tubulin-based microtentacles in detached breast tumor cells
Abstract
Solid tumor metastasis often involves detachment of epithelial carcinoma cells into the vasculature or lymphatics. However, most studies of cytoskeletal rearrangement in solid tumors focus on attached cells. In this study, we report for the first time that human breast tumor cells produce unique tubulin-based protrusions when detached from extracellular matrix. Tumor cell lines of high metastatic potential show significantly increased extension and frequency of microtubule protrusions, which we have termed tubulin microtentacles. Our previous studies in nontumorigenic mammary epithelial cells showed that such detachment-induced microtentacles are enriched in detyrosinated alpha-tubulin. However, amounts of detyrosinated tubulin were similar in breast tumor cell lines despite varying microtentacle levels. Because detyrosinated alpha-tubulin associates strongly with intermediate filament proteins, we examined the contribution of cytokeratin and vimentin filaments to tumor cell microtentacles. Increased microtentacle frequency and extension correlated strongly with loss of cytokeratin expression and up-regulation of vimentin, as is often observed during tumor progression. Moreover, vimentin filaments coaligned with microtentacles, whereas cytokeratin did not. Disruption of vimentin with PP1/PP2A-specific inhibitors significantly reduced microtentacles and inhibited cell reattachment to extracellular matrix. Furthermore, expression of a dominant-negative vimentin mutant disrupted endogenous vimentin filaments and significantly reduced microtentacles, providing specific genetic evidence that vimentin supports microtentacles. Our results define a novel model in which coordination of vimentin and detyrosinated microtubules provides structural support for the extensive microtentacles observed in detached tumor cells and a possible mechanism to promote successful metastatic spread.
Figures




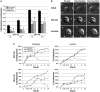
 ) or Cal-A (5nM,
) or Cal-A (5nM,  ) then suspended in DMEM −/+ LA (5μM) in combination with respective pretreatment. Blind microtentacle counts were performed following 30min suspension. Each bar represents the +S.D. for three experiments in which at least 100 GFP-Mem+ cells were counted. Pretreatment with both inhibitors and subsequent suspension in the same treatment show a statistically significant reduction in microtentacles in a cell population compared to the untreated counterpart (P<0.05, t-test, black asterisks). Suspension of cells in the presence of LA also show decrease in microtentacle frequency in combination with PP1/PP2A inhibitors (P<0.05, t-test, black asterisks). (B) DIC timecourse of transiently attached MDA-MB-436 displaying motile membrane microtentacles captured over 30min timecourse to observe effects of PP1/PP2A inhibitors on microtentacles (white arrows). (C) Disruption of vimentin by PP1/PP2A inhibitors affects cell attachment to surfaces. MCF10A and MDA-MB-436 were left untreated (
) then suspended in DMEM −/+ LA (5μM) in combination with respective pretreatment. Blind microtentacle counts were performed following 30min suspension. Each bar represents the +S.D. for three experiments in which at least 100 GFP-Mem+ cells were counted. Pretreatment with both inhibitors and subsequent suspension in the same treatment show a statistically significant reduction in microtentacles in a cell population compared to the untreated counterpart (P<0.05, t-test, black asterisks). Suspension of cells in the presence of LA also show decrease in microtentacle frequency in combination with PP1/PP2A inhibitors (P<0.05, t-test, black asterisks). (B) DIC timecourse of transiently attached MDA-MB-436 displaying motile membrane microtentacles captured over 30min timecourse to observe effects of PP1/PP2A inhibitors on microtentacles (white arrows). (C) Disruption of vimentin by PP1/PP2A inhibitors affects cell attachment to surfaces. MCF10A and MDA-MB-436 were left untreated ( ) or pretreated for 1h in Cal-A (5nM,
) or pretreated for 1h in Cal-A (5nM,  ) or OKA (1μM,
) or OKA (1μM,  ). Cells were detached and suspended over uncoated tissue culture plates or laminin coated plates in the presence of the respective PP1/PP2A inhibitor pretreatment. Reattachment efficiency was measured by XTT viability at 24h post-detachment. Values represent mean +S.D. between triplicate wells of the % cells attached in each well. Three independent experiments were performed showing similar attachment curves.
). Cells were detached and suspended over uncoated tissue culture plates or laminin coated plates in the presence of the respective PP1/PP2A inhibitor pretreatment. Reattachment efficiency was measured by XTT viability at 24h post-detachment. Values represent mean +S.D. between triplicate wells of the % cells attached in each well. Three independent experiments were performed showing similar attachment curves.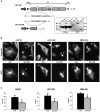
References
-
- Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. - PubMed
-
- Wittekind C, Neid M. Cancer invasion and metastasis. Oncology. 2005;69 (Suppl 1):14–6. - PubMed
-
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–95. - PubMed
-
- Wolf K, Friedl P. Molecular mechanisms of cancer cell invasion and plasticity. Br J Dermatol. 2006;154 (Suppl 1):11–5. - PubMed
-
- Yamauchi K, Yang M, Jiang P, et al. Real-time in vivo dual-color imaging of intracapillary cancer cell and nucleus deformation and migration. Cancer Res. 2005;65:4246–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

