Identification of a novel subgroup of melanomas with KIT/cyclin-dependent kinase-4 overexpression
- PMID: 18632627
- PMCID: PMC2615688
- DOI: 10.1158/0008-5472.CAN-08-0235
Identification of a novel subgroup of melanomas with KIT/cyclin-dependent kinase-4 overexpression
Abstract
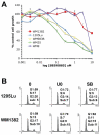
Although many melanomas harbor either activating mutations in BRAF or NRAS, there remains a substantial, yet little known, group of tumors without either mutation. Here, we used a genomic strategy to define a novel group of melanoma cell lines with co-overexpression of cyclin-dependent kinase 4 (CDK4) and KIT. Although this subgroup lacked any known KIT mutations, they had high phospho-KIT receptor expression, indicating receptor activity. Quantitative PCR confirmed the existence of a similar KIT/CDK4 subgroup in human melanoma samples. Pharmacologic studies showed the KIT/CDK4-overexpressing subgroup to be resistant to BRAF inhibitors but sensitive to imatinib in both in vitro and in vivo melanoma models. Mechanistically, imatinib treatment led to increased apoptosis and G(1) phase cell cycle arrest associated with the inhibition of phospho-ERK and increased expression of p27(KIP). Other melanoma cell lines, which retained some KIT expression but lacked phospho-KIT, were not sensitive to imatinib, suggesting that KIT expression alone is not predictive of response. We suggest that co-overexpression of KIT/CDK4 is a potential mechanism of oncogenic transformation in some BRAF/NRAS wild-type melanomas. This group of melanomas may be a subpopulation for which imatinib or other KIT inhibitors may constitute optimal therapy.
Figures




References
-
- Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–7. - PubMed
-
- Smalley KSM. A pivotal role for ERK in the oncogenic behaviour of malignant melanoma? International Journal of Cancer. 2003;104:527–32. - PubMed
-
- Verhaegen M, Bauer JA, Martin de la Vega C, et al. A novel BH3 mimetic reveals a mitogen-activated protein kinase-dependent mechanism of melanoma cell death controlled by p53 and reactive oxygen species. Cancer Res. 2006;66:11348–59. - PubMed
-
- Smalley KS, Haass NK, Brafford PA, Lioni M, Flaherty KT, Herlyn M. Multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases. Mol Cancer Ther. 2006;5:1136–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM071695/GM/NIGMS NIH HHS/United States
- CA117881/CA/NCI NIH HHS/United States
- CA47159/CA/NCI NIH HHS/United States
- CA93372/CA/NCI NIH HHS/United States
- P30 CA010815/CA/NCI NIH HHS/United States
- CA80999/CA/NCI NIH HHS/United States
- P01 CA098101/CA/NCI NIH HHS/United States
- P30 CA016520/CA/NCI NIH HHS/United States
- R01 GM071695/GM/NIGMS NIH HHS/United States
- CA25874/CA/NCI NIH HHS/United States
- R01 CA047159/CA/NCI NIH HHS/United States
- CA098101/CA/NCI NIH HHS/United States
- R01 CA118871/CA/NCI NIH HHS/United States
- P01 CA025874/CA/NCI NIH HHS/United States
- P50 CA093372/CA/NCI NIH HHS/United States
- CA10815/CA/NCI NIH HHS/United States
- R01 CA076674/CA/NCI NIH HHS/United States
- CA76674/CA/NCI NIH HHS/United States
- P01 CA114046/CA/NCI NIH HHS/United States
- R01 CA080999/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

