A new iron-oxidizing/O2-reducing supercomplex spanning both inner and outer membranes, isolated from the extreme acidophile Acidithiobacillus ferrooxidans
- PMID: 18632666
- PMCID: PMC3258861
- DOI: 10.1074/jbc.M802496200
A new iron-oxidizing/O2-reducing supercomplex spanning both inner and outer membranes, isolated from the extreme acidophile Acidithiobacillus ferrooxidans
Abstract
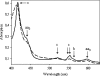
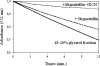
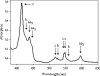
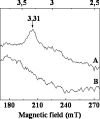
The iron respiratory chain of the acidophilic bacterium Acidithiobacillus ferrooxidans involves various metalloenzymes. Here we demonstrate that the oxygen reduction pathway from ferrous iron (named downhill pathway) is organized as a supercomplex constituted of proteins located in the outer and inner membranes as well as in the periplasm. For the first time, the outer membrane-bound cytochrome c Cyc2 was purified, and we showed that it is responsible for iron oxidation and determined that its redox potential is the highest measured to date for a cytochrome c. The organization of metalloproteins inside the supramolecular structure was specified by protein-protein interaction experiments. The isolated complex spanning the two membranes had iron oxidase as well as oxygen reductase activities, indicating functional electron transfer between the first iron electron acceptor, Cyc2, and the Cu(A) center of cytochrome c oxidase aa(3). This is the first characterization of a respirasome from an acidophilic bacterium. In Acidithiobacillus ferrooxidans,O(2) reduction from ferrous iron must be coupled to the energy-consuming reduction of NAD(+)(P) from ferrous iron (uphill pathway) required for CO(2) fixation and other anabolic processes. Besides the proteins involved in the O(2) reduction, there were additional proteins in the supercomplex, involved in uphill pathway (bc complex and cytochrome Cyc(42)), suggesting a possible physical link between these two pathways.
Figures




References
-
- Croal, L. R., Gralnick, J. A., Malasarn, D., and Newman, D. K. (2007) Annu. Rev. Genet. 38 175–202 - PubMed
-
- Rohwerder, T., Gehrke, T., Kinzler, K., and Sand, W. (2003) Appl. Microbiol. Biotechnol. 63 239–248 - PubMed
-
- Brasseur, G., Levican, G., Bonnefoy, V., Holmes, D. H., Jedlicki, E., and Lemesle-Meunier, D. (2004) Biochim. Biophys. Acta 1656 114–126 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

