Integrin VLA-4 enhances sialyl-Lewisx/a-negative melanoma adhesion to and extravasation through the endothelium under low flow conditions
- PMID: 18632734
- PMCID: PMC2544447
- DOI: 10.1152/ajpcell.00245.2008
Integrin VLA-4 enhances sialyl-Lewisx/a-negative melanoma adhesion to and extravasation through the endothelium under low flow conditions
Abstract
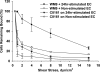
During their passage through the circulatory system, tumor cells undergo extensive interactions with various host cells including endothelial cells. The capacity of tumor cells to form metastasis is related to their ability to interact with and extravasate through endothelial cell layers, which involves multiple adhesive interactions between tumor cells and endothelium (EC). Thus it is essential to identify the adhesive receptors on the endothelial and melanoma surface that mediate those specific adhesive interactions. P-selectin and E-selectin have been reported as adhesion molecules that mediate the cell-cell interaction of endothelial cells and melanoma cells. However, not all melanoma cells express ligands for selectins. In this study, we elucidated the molecular constituents involved in the endothelial adhesion and extravasation of sialyl-Lewis(x/a)-negative melanoma cell lines under flow in the presence and absence of polymorphonuclear neutrophils (PMNs). Results show the interactions of alpha(4)beta(1) (VLA-4) on sialyl-Lewis(x/a)-negative melanoma cells and vascular adhesion molecule (VCAM-1) on inflamed EC supported melanoma adhesion to and subsequent extravasation through the EC in low shear flow. These findings provide clear evidence for a direct role of the VLA-4/VCAM-1 pathway in melanoma cell adhesion to and extravasation through the vascular endothelium in a shear flow. PMNs facilitated melanoma cell extravasation under both low and high shear conditions via the involvement of distinct molecular mechanisms. In the low shear regime, beta(2)-integrins were sufficient to enhance melanoma cell extravasation, whereas in the high shear regime, selectin ligands and beta(2)-integrins on PMNs were necessary for facilitating the melanoma extravasation process.
Figures



References
-
- Albeda SM, Mette SA, Elder DE, Stewart R, Damajanovich L, Herlyn M, Buck CA. Integrin distriution in malignant melanoma: association of the β3 subunit with tumor progression. Cancer Res 50: 6757–6764, 1990. - PubMed
-
- Alon R, Feigelson S. From rolling to arrest on blood vessels: leukocyte tap dancing on endothelial integrin ligands and chemokines at sub-second contacts. Semin Immunol 14: 93–104, 2002. - PubMed
-
- Auerbach R, Lu WC, Pardon E, Gumkowski F, Kaminska G, Kaminski A. Specificity of adhesion between murine tumor cells and capillary endothelium: an in vitro correlate of preferential metastasis in vitro. Cancer Res 47: 1492–1497, 1987. - PubMed
-
- Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hassien SR, Nelson RD, Berg EL, Erlandsen SL, Butcher EC. α4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell 80: 413–422, 1995. - PubMed
-
- Chambers AF, Naumov GN, Varghese HJ, Nadkarni KV, MacDonald IC, Groom AC. Critical steps in hematogenous metastasis: an overview. Surg Oncol Clin N Am 10: 243–255, 2001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

