The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals
- PMID: 18632739
- PMCID: PMC2638813
- DOI: 10.1093/cercor/bhn113
The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals
Abstract
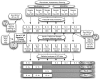
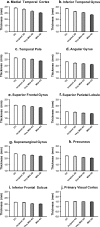
Alzheimer's disease (AD) is associated with neurodegeneration in vulnerable limbic and heteromodal regions of the cerebral cortex, detectable in vivo using magnetic resonance imaging. It is not clear whether abnormalities of cortical anatomy in AD can be reliably measured across different subject samples, how closely they track symptoms, and whether they are detectable prior to symptoms. An exploratory map of cortical thinning in mild AD was used to define regions of interest that were applied in a hypothesis-driven fashion to other subject samples. Results demonstrate a reliably quantifiable in vivo signature of abnormal cortical anatomy in AD, which parallels known regional vulnerability to AD neuropathology. Thinning in vulnerable cortical regions relates to symptom severity even in the earliest stages of clinical symptoms. Furthermore, subtle thinning is present in asymptomatic older controls with brain amyloid binding as detected with amyloid imaging. The reliability and clinical validity of AD-related cortical thinning suggests potential utility as an imaging biomarker. This "disease signature" approach to cortical morphometry, in which disease effects are mapped across the cortical mantle and then used to define ROIs for hypothesis-driven analyses, may provide a powerful methodological framework for studies of neuropsychiatric diseases.
Figures





References
-
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb Cortex. 1991;1:103–116. - PubMed
-
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. - PubMed
-
- Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer's disease. Neurology. 1992;42:1681–1688. - PubMed
-
- Atri A, Locascio JJ, Lin JM, Yap L, Dickerson BC, Grodstein F, Irizarry MC, Growdon JH, Greenberg SM. Prevalence and effects of lobar microhemorrhages in early-stage dementia. Neurodegener Dis. 2005;2:305–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

