AMPA receptor-dependent H2O2 generation in striatal medium spiny neurons but not dopamine axons: one source of a retrograde signal that can inhibit dopamine release
- PMID: 18632893
- PMCID: PMC2544473
- DOI: 10.1152/jn.90548.2008
AMPA receptor-dependent H2O2 generation in striatal medium spiny neurons but not dopamine axons: one source of a retrograde signal that can inhibit dopamine release
Abstract
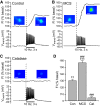
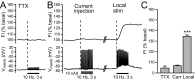
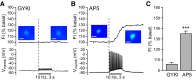
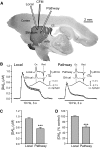
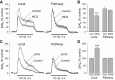
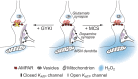
Dopamine-glutamate interactions in the striatum are critical for normal basal ganglia-mediated control of movement. Although regulation of glutamatergic transmission by dopamine is increasingly well understood, regulation of dopaminergic transmission by glutamate remains uncertain given the apparent absence of ionotropic glutamate receptors on dopaminergic axons in dorsal striatum. Indirect evidence suggests glutamatergic regulation of striatal dopamine release is mediated by a diffusible messenger, hydrogen peroxide (H2O2), generated downstream from glutamatergic AMPA receptors (AMPARs). The mechanism of H2O2-dependent inhibition of dopamine release involves activation of ATP-sensitive K+ (KATP) channels. However, the source of modulatory H2O2 is unknown. Here, we used whole cell recording, fluorescence imaging of H2O2, and voltammetric detection of evoked dopamine release in guinea pig striatal slices to examine contributions from medium spiny neurons (MSNs), the principal neurons of striatum, and dopamine axons to AMPAR-dependent H2O2 generation. Imaging studies of H2O2 generation in MSNs provide the first demonstration of AMPAR-dependent H2O2 generation in neurons in the complex brain-cell microenvironment of brain slices. Stimulation-induced increases in H2O2 in MSNs were prevented by GYKI-52466, an AMPAR antagonist, or catalase, an H2O2 metabolizing enzyme, but amplified by mercaptosuccinate (MCS), a glutathione peroxidase inhibitor. By contrast, dopamine release evoked by selective stimulation of dopamine axons was unaffected by GYKI-52466 or MCS, arguing against dopamine axons as a significant source of modulatory H2O2. Together, these findings suggest that glutamatergic regulation of dopamine release via AMPARs is mediated through retrograde signaling by diffusible H2O2 generated in striatal cells, including medium spiny neurons, rather than in dopamine axons.
Figures

References
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366–375, 1989. - PubMed
-
- Avshalumov MV, Bao L, Patel JC, Rice ME. H2O2 signaling in the nigrostriatal dopamine pathway via ATP-sensitive potassium channels: issues and answers. Antioxid Redox Signal 9: 219–231, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

