Dual regulation of Mad2 localization on kinetochores by Bub1 and Dam1/DASH that ensure proper spindle interaction
- PMID: 18632983
- PMCID: PMC2526710
- DOI: 10.1091/mbc.e08-03-0298
Dual regulation of Mad2 localization on kinetochores by Bub1 and Dam1/DASH that ensure proper spindle interaction
Abstract
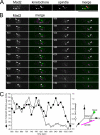
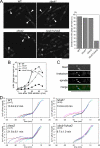
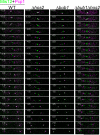
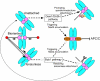
The spindle assembly checkpoint monitors the state of spindle-kinetochore interaction to prevent premature onset of anaphase. Although checkpoint proteins, such as Mad2, are localized on kinetochores that do not interact properly with the spindle, it remains unknown how the checkpoint proteins recognize abnormalities in spindle-kinetochore interaction. Here, we report that Mad2 localization on kinetochores in fission yeast is regulated by two partially overlapping but distinct pathways: the Dam1/DASH and the Bub1 pathways. We show that Mad2 is localized on "unattached" as well as "tensionless" kinetochores. Our observations suggest that Bub1 is required for Mad2 to detect tensionless kinetochores, whereas Dam1/DASH is crucial for Mad2 to detect unattached kinetochores. In cells lacking both Bub1 and Dam1/DASH, Mad2 localization on kinetochores is diminished, and mitotic progression appears to be accelerated despite the frequent occurrence of abnormal chromosome segregation. Furthermore, we found that Dam1/DASH is required for promotion of spindle association with unattached kinetochores. In contrast, there is accumulating evidence that Bub1 is involved in resolution of erroneous spindle attachment on tensionless kinetochores. These pathways may act as molecular sensors determining the state of spindle association on each kinetochore, enabling proper regulation of the checkpoint activation as well as promotion/resolution of spindle attachment.
Figures


Similar articles
-
Ringing the changes: emerging roles for DASH at the kinetochore-microtubule Interface.Chromosome Res. 2011 Apr;19(3):393-407. doi: 10.1007/s10577-011-9185-8. Chromosome Res. 2011. PMID: 21271286 Review.
-
Spindle checkpoint signaling requires the mis6 kinetochore subcomplex, which interacts with mad2 and mitotic spindles.Mol Biol Cell. 2005 Aug;16(8):3666-77. doi: 10.1091/mbc.e05-01-0014. Epub 2005 Jun 1. Mol Biol Cell. 2005. PMID: 15930132 Free PMC article.
-
Centromere-tethered Mps1 pombe homolog (Mph1) kinase is a sufficient marker for recruitment of the spindle checkpoint protein Bub1, but not Mad1.Proc Natl Acad Sci U S A. 2012 Jan 3;109(1):209-14. doi: 10.1073/pnas.1114647109. Epub 2011 Dec 19. Proc Natl Acad Sci U S A. 2012. PMID: 22184248 Free PMC article.
-
Mad1 contribution to spindle assembly checkpoint signalling goes beyond presenting Mad2 at kinetochores.EMBO Rep. 2014 Mar;15(3):291-8. doi: 10.1002/embr.201338114. Epub 2014 Jan 29. EMBO Rep. 2014. PMID: 24477934 Free PMC article.
-
Bub1 and BubR1: at the interface between chromosome attachment and the spindle checkpoint.Mol Cell Biol. 2011 Aug;31(15):3085-93. doi: 10.1128/MCB.05326-11. Epub 2011 May 31. Mol Cell Biol. 2011. PMID: 21628528 Free PMC article. Review.
Cited by
-
KSHV LANA--the master regulator of KSHV latency.Viruses. 2014 Dec 11;6(12):4961-98. doi: 10.3390/v6124961. Viruses. 2014. PMID: 25514370 Free PMC article. Review.
-
Ringing the changes: emerging roles for DASH at the kinetochore-microtubule Interface.Chromosome Res. 2011 Apr;19(3):393-407. doi: 10.1007/s10577-011-9185-8. Chromosome Res. 2011. PMID: 21271286 Review.
-
Elastic Tethers Between Separating Anaphase Chromosomes Regulate the Poleward Speeds of the Attached Chromosomes in Crane-Fly Spermatocytes.Front Mol Biosci. 2020 Jul 29;7:161. doi: 10.3389/fmolb.2020.00161. eCollection 2020. Front Mol Biosci. 2020. PMID: 32850955 Free PMC article.
-
The kinetochore protein Kis1/Eic1/Mis19 ensures the integrity of mitotic spindles through maintenance of kinetochore factors Mis6/CENP-I and CENP-A.PLoS One. 2014 Nov 6;9(11):e111905. doi: 10.1371/journal.pone.0111905. eCollection 2014. PLoS One. 2014. PMID: 25375240 Free PMC article.
-
Tripeptidyl peptidase II in human oral squamous cell carcinoma.J Cancer Res Clin Oncol. 2013 Jan;139(1):123-30. doi: 10.1007/s00432-012-1307-y. Epub 2012 Sep 18. J Cancer Res Clin Oncol. 2013. PMID: 22986808 Free PMC article.
References
-
- Andrews P. D., Ovechkina Y., Morrice N., Wagenbach M., Duncan K., Wordeman L., Swedlow J. R. Aurora B regulates MCAK at the mitotic centromere. Dev. Cell. 2004;6:253–268. - PubMed
-
- Aoki K., Nakaseko Y., Kinoshita K., Goshima G., Yanagida M. CDC2 phosphorylation of the fission yeast dis1 ensures accurate chromosome segregation. Curr. Biol. 2006;16:1627–1635. - PubMed
-
- Chen R. H., Waters J. C., Salmon E. D., Murray A. W. Association of spindle assembly checkpoint component XMAD2 with unattached kinetochores. Science. 1996;274:242–246. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

