Integrin-associated protein association with SRC homology 2 domain containing tyrosine phosphatase substrate 1 regulates igf-I signaling in vivo
- PMID: 18633106
- PMCID: PMC2551672
- DOI: 10.2337/db08-0326
Integrin-associated protein association with SRC homology 2 domain containing tyrosine phosphatase substrate 1 regulates igf-I signaling in vivo
Abstract
Objective: Smooth muscle cell (SMC) maintained in medium containing normal levels of glucose do not proliferate in response to IGF-I, whereas cells maintained in medium containing 25 mmol/l glucose can respond. The aim of this study was to determine whether signaling events that have been shown to be required for stimulation of SMC growth were regulated by glucose concentrations in vivo.
Research design and methods: We compared IGF-I-stimulated signaling events and growth in the aortic smooth muscle cells from normal and hyperglycemic mice.
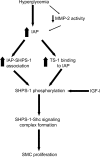
Results: We determined that, in mice, hyperglycemia was associated with an increase in formation of the integrin-associated protein (IAP)/Src homology 2 domaine containing tyrosine phosphatase substrate 1 (SHPS-1) complex. There was a corresponding increase in Shc recruitment to SHPS-1 and Shc phosphorylation in response to IGF-I. There was also an increase in mitogen-activated protein kinase activation and SMC proliferation. The increase in IAP association with SHPS-1 in hyperglycemia appeared to be due to the protection of IAP from cleavage that occurred during exposure to normal glucose. In addition, we demonstrated that the protease responsible for IAP cleavage was matrix metalloprotease-2. An anti-IAP antibody that disrupted the IAP-SHPS-1 association resulted in complete inhibition of IGF-I-stimulated proliferation.
Conclusions: Taken together, our results support a model in which hyperglycemia is associated with a reduction in IAP cleavage, thus allowing the formation of the IAP-SHPS-1 signaling complex that is required for IGF-I-stimulated proliferation of SMC.
Figures



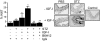
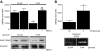
References
-
- Kannel WB, McGee DL: Diabetes and cardiovascular risk factors: the Framingham study. Circulation 59:8–13, 1979 - PubMed
-
- Diabetes Control and Complications Trial Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986, 1993 - PubMed
-
- Ross R: The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362:801–809, 1993 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

