Alterations in microRNA expression contribute to fatty acid-induced pancreatic beta-cell dysfunction
- PMID: 18633110
- PMCID: PMC2551683
- DOI: 10.2337/db07-1252
Alterations in microRNA expression contribute to fatty acid-induced pancreatic beta-cell dysfunction
Abstract
Objective: Visceral obesity and elevated plasma free fatty acids are predisposing factors for type 2 diabetes. Chronic exposure to these lipids is detrimental for pancreatic beta-cells, resulting in reduced insulin content, defective insulin secretion, and apoptosis. We investigated the involvement in this phenomenon of microRNAs (miRNAs), a class of noncoding RNAs regulating gene expression by sequence-specific inhibition of mRNA translation.
Research design and methods: We analyzed miRNA expression in insulin-secreting cell lines or pancreatic islets exposed to palmitate for 3 days and in islets from diabetic db/db mice. We studied the signaling pathways triggering the changes in miRNA expression and determined the impact of the miRNAs affected by palmitate on insulin secretion and apoptosis.
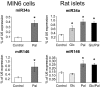
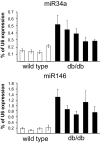
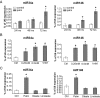
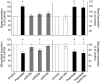
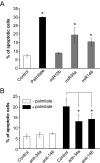
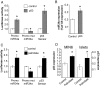
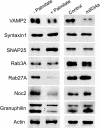
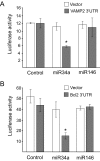
Results: Prolonged exposure of the beta-cell line MIN6B1 and pancreatic islets to palmitate causes a time- and dose-dependent increase of miR34a and miR146. Elevated levels of these miRNAs are also observed in islets of diabetic db/db mice. miR34a rise is linked to activation of p53 and results in sensitization to apoptosis and impaired nutrient-induced secretion. The latter effect is associated with inhibition of the expression of vesicle-associated membrane protein 2, a key player in beta-cell exocytosis. Higher miR146 levels do not affect the capacity to release insulin but contribute to increased apoptosis. Treatment with oligonucleotides that block miR34a or miR146 activity partially protects palmitate-treated cells from apoptosis but is insufficient to restore normal secretion.
Conclusions: Our findings suggest that at least part of the detrimental effects of palmitate on beta-cells is caused by alterations in the level of specific miRNAs.
Figures
References
-
- Weir GC, Bonner-Weir S: Five stages of evolving β-cell dysfunction during progression to diabetes. Diabetes 53 (Suppl. 3): S16–S21, 2004 - PubMed
-
- Newsholme P, Keane D, Welters HJ, Morgan NG: Life and death decisions of the pancreatic beta-cell: the role of fatty acids. Clin Sci (Lond) 112: 27–42, 2007 - PubMed
-
- Bushati N, Cohen SM: microRNA Functions. Annu Rev Cell Dev Biol 23: 175–205, 2007 - PubMed
-
- Bartel DP: MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297, 2004 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

