NMDA receptors in clinical neurology: excitatory times ahead
- PMID: 18635022
- PMCID: PMC3589564
- DOI: 10.1016/S1474-4422(08)70165-0
NMDA receptors in clinical neurology: excitatory times ahead
Abstract
Since the N-methyl-D-aspartate receptor (NMDAR) subunits were cloned less than two decades ago, a substantial amount of research has been invested into understanding their physiological function in the healthy CNS. Research has also been directed at their pathological roles in various neurological diseases, including disorders resulting from acute excitotoxic insults (eg, ischaemic stroke, traumatic brain injury), diseases due to chronic neurodegeneration (eg, Alzheimer's, Parkinson's, and Huntington's diseases and amyotrophic lateral sclerosis), disorders arising from sensitisation of neurons (eg, epilepsy, neuropathic pain), and neurodevelopmental disorders associated with NMDAR hypofunction (eg, schizophrenia). Selective NMDAR antagonists have not produced positive results in clinical trials. However, there are other NMDAR-targeted therapies used in current practice that are effective for treating some neurological disorders. In this Review, we describe the evidence for the use of these therapies and provide an overview of drugs being investigated in clinical trials. We also discuss new NMDAR-targeted strategies in clinical neurology.
Conflict of interest statement
LVK and SKK have no conflicts of interest to declare. MWS has been a consultant for various pharmaceutical companies and a member of scientific advisory boards. MWS has received speaker’s fees and participated in meetings supported by unrestricted grants from industry. None of these declarations present a conflict of interest in relation to the content of this Review.
Figures
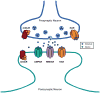
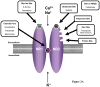

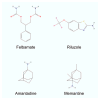

References
-
- Pinheiro PS, Mulle C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nat Rev Neurosci. 2008 Jun;9(6):423–36. - PubMed
-
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999 Mar;51(1):7–61. - PubMed
-
- Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007 Feb;7(1):39–47. - PubMed
-
- Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991 Nov 7;354(6348):31–7. - PubMed
-
- Meguro H, Mori H, Araki K, Kushiya E, Kutsuwada T, Yamazaki M, et al. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature. 1992 May 7;357(6373):70–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

