Sex chromosome complement affects nociception and analgesia in newborn mice
- PMID: 18635401
- PMCID: PMC2575001
- DOI: 10.1016/j.jpain.2008.06.001
Sex chromosome complement affects nociception and analgesia in newborn mice
Abstract
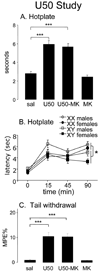
In animal studies of nociception, females are often more sensitive to painful stimuli, whereas males are often more sensitive to analgesia induced by mu-agonists. Sex differences are found even at birth, and in adulthood are likely caused, at least in part, by differences in levels of gonadal hormones. In this report, we investigate nociception and analgesia in neonatal mice and assess the contribution of the direct action of sex chromosome genes in hotplate and tail withdrawal tests. We used the 4 core genotypes mouse model, in which gonadal sex is independent of the complement of sex chromosomes (XX vs XY). Mice were tested at baseline and then injected with mu-opioid agonist morphine (10 mg/kg) or with the kappa-opioid agonist U50,488H (U50, 12.5 mg/kg) with or without the N-methyl-D-aspartate (NMDA) receptor antagonist MK-801 (0.1 mg/kg). On the day of birth, XX mice showed faster baseline latencies than XY in tail withdrawal, irrespective of their gonadal type. Gonadal males showed greater effects of morphine than gonadal females in the hotplate test, irrespective of their sex chromosome complement. U50 and morphine were effective analgesics in both tests, but MK-801 did not block the U50 effect. The results suggest that sex chromosome complement and gonadal secretions both contribute to sex differences in nociception and analgesia by the day of birth.
Perspective: Sex differences in pain may stem not only from the action of gonadal hormones on pain circuits but from the sex-specific action of X and Y genes. Identification of sex chromosome genes causing sex differences could contribute to better pain therapy in females and males.
Figures


Similar articles
-
Sex chromosome complement affects nociception in tests of acute and chronic exposure to morphine in mice.Horm Behav. 2008 Jan;53(1):124-30. doi: 10.1016/j.yhbeh.2007.09.003. Epub 2007 Sep 14. Horm Behav. 2008. PMID: 17956759 Free PMC article.
-
dextro- and levo-morphine attenuate opioid delta and kappa receptor agonist produced analgesia in mu-opioid receptor knockout mice.Eur J Pharmacol. 2006 Feb 15;531(1-3):103-7. doi: 10.1016/j.ejphar.2005.12.012. Epub 2006 Jan 30. Eur J Pharmacol. 2006. PMID: 16445907
-
Qualitative sex differences in kappa-opioid analgesia in mice are dependent on age.Neurosci Lett. 2004 Jun 10;363(2):178-81. doi: 10.1016/j.neulet.2004.04.004. Neurosci Lett. 2004. PMID: 15172110
-
What does the "four core genotypes" mouse model tell us about sex differences in the brain and other tissues?Front Neuroendocrinol. 2009 Jan;30(1):1-9. doi: 10.1016/j.yfrne.2008.11.001. Epub 2008 Nov 11. Front Neuroendocrinol. 2009. PMID: 19028515 Free PMC article. Review.
-
Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues.J Neuroendocrinol. 2009 Mar;21(4):377-86. doi: 10.1111/j.1365-2826.2009.01831.x. J Neuroendocrinol. 2009. PMID: 19207816 Free PMC article. Review.
Cited by
-
Sex differences in kappa opioid pharmacology.Life Sci. 2011 Jan 3;88(1-2):2-16. doi: 10.1016/j.lfs.2010.10.007. Epub 2010 Oct 14. Life Sci. 2011. PMID: 20951148 Free PMC article. Review.
-
Sex differences in partner preferences in humans and animals.Philos Trans R Soc Lond B Biol Sci. 2016 Feb 19;371(1688):20150118. doi: 10.1098/rstb.2015.0118. Epub 2016 Feb 1. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 26833838 Free PMC article. Review.
-
IS MALE BRAIN DIFFERENT FROM FEMALE BRAIN?Slov Vet Zb. 2009 Sep 1;46(3):85-91. Slov Vet Zb. 2009. PMID: 20729972 Free PMC article.
-
Reframing sexual differentiation of the brain.Nat Neurosci. 2011 Jun;14(6):677-83. doi: 10.1038/nn.2834. Epub 2011 May 25. Nat Neurosci. 2011. PMID: 21613996 Free PMC article. Review.
-
Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon.Nat Rev Neurosci. 2012 Dec;13(12):859-66. doi: 10.1038/nrn3360. Nat Rev Neurosci. 2012. PMID: 23165262 Review.
References
-
- Arnold AP. Concepts of genetic and hormonal induction of vertebrate sexual differentiation in the twentieth century, with special reference to the brain. In: Pfaff DW, Arnold AP, Etgen A, Fahrbach S, Rubin R, editors. Hormones, Brain, and Behavior. Academic Press; 2002. pp. 105–135.
-
- Arnold AP. Sex chromosomes and brain gender. Nature Reviews Neuroscience. 2004;5:701–708. - PubMed
-
- Arnold AP, Burgoyne PS. Are XX and XY brain cells intrinsically different? Trends in Endocrinology and Metabolism. 2004;15:6–11. - PubMed
-
- Barr GA, Limon E, Luthmann RA, Barr GA, Cheng J, Wang S. Analgesia induced by local plantar injections of opiates in the formalin test in infant rats. Dev Psychobiol. 2003;42:111–122. - PubMed
-
- Berkley KJ. Sex differences in pain. Behavioral and Brain Sciences. 1997;20:371–380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

