Long patch base excision repair in mammalian mitochondrial genomes
- PMID: 18635552
- PMCID: PMC2546560
- DOI: 10.1074/jbc.M803491200
Long patch base excision repair in mammalian mitochondrial genomes
Abstract
The mitochondrial genome is highly susceptible to damage by reactive oxygen species (ROS) generated endogenously as a byproduct of respiration. ROS-induced DNA lesions, including oxidized bases, abasic (AP) sites, and oxidized AP sites, cause DNA strand breaks and are repaired via the base excision repair (BER) pathway in both the nucleus and mitochondria. Repair of damaged bases and AP sites involving 1-nucleotide incorporation, named single nucleotide (SN)-BER, was observed with mitochondrial and nuclear extracts. During SN-BER, the 5'-phosphodeoxyribose (dRP) moiety, generated by AP-endonuclease (APE1), is removed by the lyase activity of DNA polymerase gamma (pol gamma) and polymerase beta in the mitochondria and nucleus, respectively. However, the repair of oxidized deoxyribose fragments at the 5' terminus after strand break would require 5'-exo/endonuclease activity that is provided by the flap endonuclease (FEN-1) in the nucleus, resulting in multinucleotide repair patch (long patch (LP)-BER). Here we show the presence of a 5'-exo/endonuclease in the mitochondrial extracts of mouse and human cells that is involved in the repair of a lyase-resistant AP site analog via multinucleotide incorporation, upstream and downstream to the lesion site. We conclude that LP-BER also occurs in the mitochondria requiring the 5'-exo/endonuclease and pol gamma with 3'-exonuclease activity. Although a FEN-1 antibody cross-reacting species was detected in the mitochondria, it was absent in the LP-BER-proficient APE1 immunocomplex isolated from the mitochondrial extract that contains APE1, pol gamma, and DNA ligase 3. The LP-BER activity was marginally affected in FEN-1-depleted mitochondrial extracts, further supporting the involvement of an unidentified 5'-exo/endonuclease in mitochondrial LP-BER.
Figures



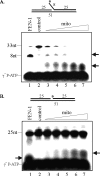
References
-
- Robin, E. D., and Wong, R. (1988) J. Cell. Physiol. 136507 –513 - PubMed
-
- Anderson, S., Bankier, A. T., Barrell, B. G., de Bruijn, M. H., Coulson, A. R., Drouin, J., Eperon, I. C., Nierlich, D. P., Roe, B. A., Sanger, F., Schreier, P. H., Smith, A. J., Staden, R., and Young, I. G. (1981) Nature 290457 –465 - PubMed
-
- Mandavilli, B. S., Santos, J. H., and Van Houten, B. (2002) Mutat. Res. 509127 –151 - PubMed
-
- Sung, J. S., and Demple, B. (2006) FEBS J. 2731620 –1629 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

