Origin of synchronized low-frequency blood oxygen level-dependent fluctuations in the primary visual cortex
- PMID: 18635612
- PMCID: PMC8118784
- DOI: 10.3174/ajnr.A1220
Origin of synchronized low-frequency blood oxygen level-dependent fluctuations in the primary visual cortex
Abstract
Background and purpose: Low-frequency (<0.08 Hz) fluctuations in spontaneous blood oxygen level-dependent (BOLD) signal intensity show synchronization across anatomically interconnected and functionally specific brain regions, suggesting a neural origin of fluctuations. To determine the mechanism by which high-frequency neural activity results in low-frequency BOLD fluctuations, I obtained measurements of the effects of neurovascular coupling on the frequency content of BOLD fluctuations.
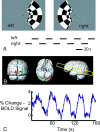
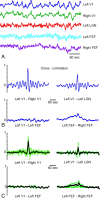
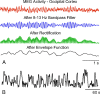
Materials and methods: 3T recordings of BOLD signal intensity in the primary visual cortex were obtained in response to visual stimuli presented at varying temporal frequencies to determine which stimulus frequencies were successfully transmitted to BOLD signal intensity. Additional BOLD time series recordings were performed in a resting state and during natural visual stimulation, and frequencies comprising BOLD fluctuations were measured. Magnetoencephalographic (MEG) time series recordings were obtained in a resting state to measure which components of MEG signal intensity best correlated in frequency distribution to observed BOLD fluctuations.
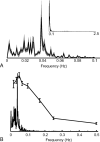
Results: Visually driven oscillations in BOLD signal intensity were observed up to 0.2 Hz, representing a mismatch between low-pass filter properties of neurovascular coupling and observed frequencies of spontaneous BOLD fluctuations, which are <0.05 Hz in the primary visual cortex. Visual stimulation frequencies of >0.2 Hz resulted in frequency-dependent increases in mean BOLD response. Amplitude modulation of high-frequency neural activity was measured in MEG time series data, which demonstrated 1/frequency distribution with the greatest power comprising frequencies <0.05 Hz, consistent with the distribution of observed BOLD fluctuations.
Conclusion: Synchronized low-frequency BOLD fluctuations likely arise from a combination of vascular low-pass filtering and low-frequency amplitude modulation of neural activity.
Figures



References
-
- Biswal B, Yetkin FZ, Haughton VM, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Mag Res Med 1995;34:537–41 - PubMed
-
- Birn RM, Diamon JB, Smith MA, et al. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage 2006;31:1536–48 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
