Activation of recombinant NR1/NR2C NMDA receptors
- PMID: 18635641
- PMCID: PMC2614009
- DOI: 10.1113/jphysiol.2008.158634
Activation of recombinant NR1/NR2C NMDA receptors
Abstract
The N-methyl-d-aspartate (NMDA) subtype of ionotropic glutamate receptors comprises both NR1 and NR2 subunits, and plays numerous roles in both physiological and pathophysiological processes in the central nervous system (CNS). NR2C-containing NMDA receptors are most abundant in cerebellum, thalamus and olfactory bulb, and are also expressed in oligodendrocytes and hippocampal interneurons. We have used patch clamp recording to explore the activation properties of recombinant NR1/NR2C receptors expressed in HEK293 cells. NR1/NR2C receptors activated by a maximally effective concentration of glutamate and glycine had two main conductance levels of 45 pS and 28 pS when the extracellular Ca(2+) concentration was 0.5 mm and the holding potential was -80 mV. The occurrence of the lower subconductance state was reduced in the absence of extracellular Ca(2+). The distribution of closed durations recorded from patches with a high probability of containing only one active channel were best fitted by five exponential functions; the apparent open duration histogram could be fitted by two exponential functions (n = 10 patches). The apparent mean open time of NR1/NR2C receptors was brief (0.52 +/- 0.04 ms), suggesting that the stability of the open state of the NR1/NR2C receptors is lower than other NR2-containing receptors. NR1/NR2C open probability was exceptionally low, being 0.011 +/- 0.002 in patches containing a single active receptor (n = 8). Fast agonist concentration jumps were performed on outside out patches with multiple NR1/NR2C channels, which activated with a 10-90% rise time of 3.9 +/- 0.4 ms, faster than other NR2-containing receptors. The deactivation time constant after a brief (5-8 ms) application of a maximally effective concentration of agonists was 319 +/- 34 ms. The majority of the patches also showed a modest level of desensitization that could be described by either a single or a double exponential time course with the fastest time constant between 15 and 47 ms. Conceptual models of activation were fitted using the maximum interval likelihood (MIL) method to the sequence of open and closed durations recorded from outside-out patches that contained one active NR1/NR2C channel. NR1/NR2C receptor properties including modest desensitization and low open probability could be described by gating schemes similar to those previously proposed for other NMDA receptor subunit combinations.
Figures
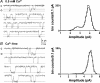
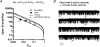

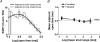
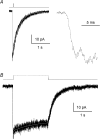


References
-
- Banke TG, Traynelis SF. Activation of NR1/NR2B NMDA receptors. Nat Neurosci. 2003;6:144–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

