Movement of the tongue during normal breathing in awake healthy humans
- PMID: 18635645
- PMCID: PMC2652195
- DOI: 10.1113/jphysiol.2008.156430
Movement of the tongue during normal breathing in awake healthy humans
Abstract
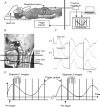
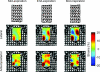
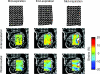
Electromyographic (EMG) activity of the airway muscles suggest that genioglossus is the primary upper airway dilator muscle. However, EMG data do not necessarily translate into tissue motion and most imaging modalities are limited to assessment of the surfaces of the upper airway. In this study, we hypothesized that genioglossus moves rhythmically during the respiratory cycle and that the motion within is inhomogeneous. A 'tagged' magnetic resonance imaging technique was used to characterize respiratory-related tissue motions around the human upper airway in quiet breathing. Motion of airway tissues at different segments of the eupnoeic respiratory cycle was imaged in six adult subjects by triggering the scanner at the end of inspiration. Displacements of the 'tags' were analysed using the harmonic phase method (HARP). Respiratory timing was monitored by a band around the upper abdomen. The genioglossus moved during the respiratory cycle. During expiration, the genioglossus moved posteriorly and during inspiration, it moved anteriorly. The degree of motion varied between subjects. The maximal anteroposterior movement of a point tracked on the genioglossus was 1.02 +/- 0.54 mm (mean +/- s.d.). The genioglossus moved over the geniohyoid muscle, with minimal movement in other muscles surrounding the airway at the level of the soft palate. Local deformation of the tongue was analysed using two-dimensional strain maps. Across the respiratory cycle, positive strains within genioglossus reached peaks of 17.5 +/- 9.3% and negative strains reached peaks of -16.3 +/- 9.3% relative to end inspiration. The patterns of strains were consistent with elongation and compression within a constant volume structure. Hence, these data suggest that even during respiration, the tongue behaves as a muscular hydrostat.
Figures



Comment in
-
Going to the movies can make all the difference.J Physiol. 2008 Sep 1;586(17):4035. doi: 10.1113/jphysiol.2008.160317. J Physiol. 2008. PMID: 18765519 Free PMC article. No abstract available.
References
-
- Armstrong JJ, Leigh MS, Sampson DD, Walsh JH, Hillman DR, Eastwood PR. Quantitative upper airway imaging with anatomic optical coherence tomography. Am J Respir Crit Care Med. 2006;173:226–233. - PubMed
-
- Bailey EF, Huang YH, Fregosi RF. Anatomic consequences of intrinsic tongue muscle activation. J Appl Physiol. 2006;101:1377–1385. - PubMed
-
- Berry RB, White DP, Roper J, Pillar G, Fogel RB, Stanchina M, Malhotra A. Awake negative pressure reflex response of the genioglossus in OSA patients and normal subjects. J Appl Physiol. 2003;94:1875–1882. - PubMed
-
- Brennick MJ, Gefter WB, Margulies SS. Mechanical effects of genioglossus muscle stimulation on the pharyngeal airway by MRI in cats. Respir Physiol Neurobiol. 2007;156:154–164. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

