Monosynaptic convergence of C- and Adelta-afferent fibres from different segmental dorsal roots on to single substantia gelatinosa neurones in the rat spinal cord
- PMID: 18635648
- PMCID: PMC2652182
- DOI: 10.1113/jphysiol.2008.154898
Monosynaptic convergence of C- and Adelta-afferent fibres from different segmental dorsal roots on to single substantia gelatinosa neurones in the rat spinal cord
Abstract
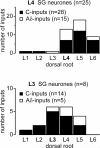
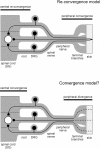
Although it is known that each spinal cord segment receives thin-fibre inputs from several segmental dorsal roots, it remains unclear how these inputs converge at the cellular level. To study whether C- and Adelta-afferents from different roots can converge monosynaptically on to a single substantia gelatinosa (SG) neurone, we performed tight-seal recordings from SG neurones in the entire lumbar enlargement of the rat spinal cord with all six segmental (L1-L6) dorsal roots attached. The neurones in the spinal cord were visualized using our recently developed oblique LED illumination technique. Individual SG neurones from the spinal segment L4 or L3 were voltage clamped to record the monosynaptic EPSCs evoked by stimulating ipsilateral L1-L6 dorsal roots. We found that one-third of the SG neurones receive simultaneous monosynaptic inputs from two to four different segmental dorsal roots. For the SG neurones from segment L4, the major monosynaptic input was from the L4-L6 roots, whereas for those located in segment L3 the input pattern was shifted to the L2-L5 roots. Based on these data, we propose a new model of primary afferent organization where several C- or Adelta-fibres innervating one cutaneous region (peripheral convergence) and ascending together in a common peripheral nerve may first diverge at the level of spinal nerves and enter the spinal cord through different segmental dorsal roots, but finally re-converge monosynaptically on to a single SG neurone. This organization would allow formation of precise and robust neural maps of the body surface at the spinal cord level.
Figures





Similar articles
-
Electrophysiological mapping of the nociceptive inputs to the substantia gelatinosa in rat horizontal spinal cord slices.J Physiol. 2004 Oct 1;560(Pt 1):303-15. doi: 10.1113/jphysiol.2004.068700. Epub 2004 Aug 5. J Physiol. 2004. PMID: 15297573 Free PMC article.
-
Cell-type-specific excitatory and inhibitory circuits involving primary afferents in the substantia gelatinosa of the rat spinal dorsal horn in vitro.J Physiol. 2007 Jun 1;581(Pt 2):603-18. doi: 10.1113/jphysiol.2006.123919. Epub 2007 Mar 8. J Physiol. 2007. PMID: 17347278 Free PMC article.
-
Synaptic responses of substantia gelatinosa neurones to dorsal column stimulation in rat spinal cord in vitro.J Physiol. 1994 Jul 1;478 ( Pt 1)(Pt 1):87-99. doi: 10.1113/jphysiol.1994.sp020232. J Physiol. 1994. PMID: 7965839 Free PMC article.
-
Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord.J Physiol. 1990 Nov;430:315-35. doi: 10.1113/jphysiol.1990.sp018293. J Physiol. 1990. PMID: 1982314 Free PMC article.
-
Primary afferent-evoked glycine- and GABA-mediated IPSPs in substantia gelatinosa neurones in the rat spinal cord in vitro.J Physiol. 1995 Jan 1;482 ( Pt 1)(Pt 1):29-38. doi: 10.1113/jphysiol.1995.sp020497. J Physiol. 1995. PMID: 7730987 Free PMC article.
Cited by
-
Unique Characteristics of the Dorsal Root Ganglion as a Target for Neuromodulation.Pain Med. 2019 Jun 1;20(Suppl 1):S23-S30. doi: 10.1093/pm/pnz012. Pain Med. 2019. PMID: 31152179 Free PMC article. Review.
-
Organization of intralaminar and translaminar neuronal connectivity in the superficial spinal dorsal horn.J Neurosci. 2009 Apr 22;29(16):5088-99. doi: 10.1523/JNEUROSCI.6175-08.2009. J Neurosci. 2009. PMID: 19386904 Free PMC article.
-
Monosynaptic convergence of somatic and visceral C-fiber afferents on projection and local circuit neurons in lamina I: a substrate for referred pain.Pain. 2015 Oct;156(10):2042-2051. doi: 10.1097/j.pain.0000000000000267. Pain. 2015. PMID: 26098437 Free PMC article.
-
Mammalian target of rapamycin signaling in the spinal cord is required for neuronal plasticity and behavioral hypersensitivity associated with neuropathy in the rat.J Pain. 2010 Dec;11(12):1356-67. doi: 10.1016/j.jpain.2010.03.013. Epub 2010 May 8. J Pain. 2010. PMID: 20452291 Free PMC article.
-
A-Type KV Channels in Dorsal Root Ganglion Neurons: Diversity, Function, and Dysfunction.Front Mol Neurosci. 2018 Aug 6;11:253. doi: 10.3389/fnmol.2018.00253. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30127716 Free PMC article. Review.
References
-
- Amaral DG. The Functional Organization of Perception and Movement. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 4th edn. McGraw-Hill, New York; 2000. pp. 337–348.
-
- Bennett GJ, Abdelmoumene M, Hayashi H, Dubner R. Physiology and morphology of substantia gelatinosa neurons intracellularly stained with horseradish peroxidase. J Comp Neurol. 1980;194:809–827. - PubMed
-
- Brown AG. Organization in the Spinal Cord. Berlin, Heidelberg: Springer-Verlag; 1981.
-
- Brown PB, Fuchs JL. Somatotopic representation of hindlimb skin in cat dorsal horn. J Neurophysiol. 1975;38:1–9. - PubMed
-
- Cervero F. Dorsal horn neurones and their sensory inputs. In: Yasksh TL, editor. Spinal Afferent Processing. New York: Plenum Press; 1987. pp. 197–216.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

